
Due diligence for responsible business conduct with
regards to people, animals, society and the environment
Account reporting year
2023
for Mediq Norge AS
Ethical Trade Norway has assessed the report of Mediq Norge AS to meet the criteria of
our Base Level. More information about our Base Level can be found here.

To Readers Of The Report
Enterprises and the public sector have a great impact on people, society, the environment, climate, and animals
and can both contribute positively to development, or negatively by causing harm. Enterprises therefore hold a
central role in achieving UN’s Sustainable Development Goals (SDGs) and the Paris Agreement’s 1,5-degree
target.
This report can be used as an account for the Transparency Act, but it has a broader scope with climate and the
environment, circular economy, and anti-corruption indicators also being included. Our members are obligated
to carry out due diligence and report annually on their work. Base level1 members also meet the Transparency
Act’s due diligence duty, and partially the Act’s information duty.
Ethical Trade Norway’s concept of responsible business conduct equals OECD’s terminology and due diligence
methodology. This is the systematic effort that enterprises do to identify, prevent, or mitigate adverse impacts
and explain how they manage their risks of negative impact, as well as provide remediation to people, animals,
society, and the environment where this is required – is called due diligence. Norwegian authorities expect all
enterprises, regardless of their size, to carry out due diligence on society, the environment, and animals in
accordance with the UN’s Guiding Principles for Business and Human Rights (UNGP) and OECD’s Guidelines for
Multinational Enterprises. This applies to enterprises, the public sector, and organisations.
Ethical Trade Norway’s Declaration of Principles (our Code of Conduct) for Responsible Business Conduct covers
the areas of decent work, human rights, environment/climate, anti-corruption, and animal welfare. This report is
done in full transparency and in line with UNGP and OECD’s guidelines. The reports of all members are publicly
accessible on Ethical Trade Norway’s website.
Heidi Furustøl
Executive Director
Ethical Trade Norway
| Mediq Norge AS | 2
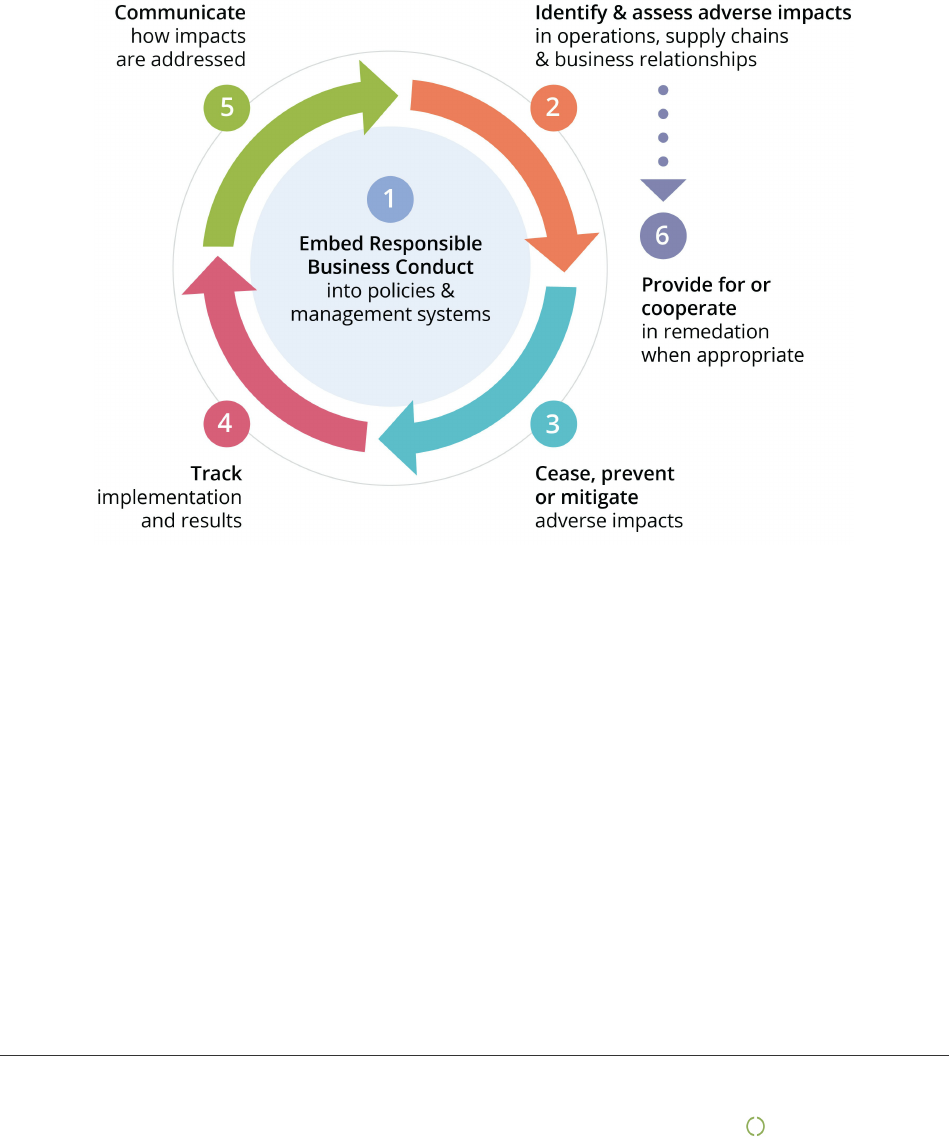
Due diligence
This report is based on the UN Guiding Principles on Business and Human Rights and the OECD model for Due
Diligence for Responsible Business Conduct.
The model has six steps that describe how companies can work for more responsible and sustainable business
practice. However, being good at due diligence does not mean no negative impact on people, planet and the
society. It means that the company is open and honest about challenges faced and shows how this is managed in
the best possible way in collaboration with its stakeholders. This report is divided in chapters following the
OECD model.
| Mediq Norge AS | 3

Preface From CEO
As a leading supplier of medical devices, Mediq Norge is naturally engaged in UN Goal 3 “Ensure healthy lives
and promote well-being for all at all ages”. In 2023 we have relaunched our ESG Strategy, we are transitioning
from a CSR-focused approach to a more encompassing ESG model. For those unfamiliar, while Corporate Social
Responsibility (CSR) has traditionally emphasized ethical operations and sustainability initiatives, the
Environmental, Social, and Governance (ESG) framework takes a broader view. ESG not only addresses the
negative impacts, but focuses on minimizing those impacts and redirecting them toward creating positive
outcomes.
While developing the new ESG strategy, we've examined the positive and negative environmental and social
footprints we leave behind.
We aligned our strategy pillars with relevant UN Sustainable Development Goals.
Now, our strategy is structured around four key pillars:
• Our products
• Our services
• Our operations
• Our people.
Our new ESG Strategy goes hand in hand with our core values:
• Caring heart
• Customer drive
• Champion spirit
We invite you to learn more about our ESG strategy through reading this report.
Joachim Warnberg
Managing Director, Mediq Norge AS
| Mediq Norge AS | 4

Board Signature
Oslo
14.02.2024
Enterprise information and enterprise context
| Mediq Norge AS | 5
Enterprise information and enterprise context
Key enterprise information
Enterprise name
Mediq Norge AS
Head office address
Brynsveien 14, 0667 Oslo
Main brands, products and services offered by the enterprise
Mediq Norge sell and service Medical Devices and consumables to both public and private institutions and
companies. We represent Mediq Own Brand products, like Klinion, Curion, Absorin and Cenaman. The Mediq
Own Brand products are manufactured by our sister company, Medeco. We also represent a number of A-brand
suppliers, such as: Werfen, KCI, Care of Sweden, Sterisol, Teleflex, Semperit, Ecolab and Boston Scientific.
| Mediq Norge AS | 6

Description of enterprise structure
Mediq Norge AS imports, markets, sells and distributes Medical Devices, as well as install, service and maintains
Medical Devices to the Norwegian market. Mediqs customers are primary healthcare service, public and private
institutions and companies. Like hospitals, healthcare institutions, general practitioners, army, police,
wholesalers, first aid dealers and retail companies.
Mediq Norge AS is part of the Mediq Group with activities in 13 countries with 3000 employees.
The Mediq Group is owned by the private equity company Advent.
Within Mediq Group, Medeco is the only company that has the Manufacturer role. Medeco is the legal
Manufacturer of the Mediq Own Brands. Medeco chooses the products, contracts third-party producers and
follows up the supply chain for the Mediq Own Brand products.
Mediq Norge acts as an Importer and Distributer of a large range of products from nearly 300 different suppliers.
Medeco being one of them.
Our ESG policy and strategy are set by Top Management in Mediq Group. Top Management are supported by
ESG committee on Group level. The ESG committee consists of Top Manager representatives from Category,
Sourcing, Supply Chain and HR. Committee is lead by Group ESG Manager. Ambitions related to PRODUCTS,
SERVICES, OPERATIONS and OUR PEOPLE are set in our ESG strategy. (Further description in 1.C.1)
Road Map 2030 are being developed to set actions in order to reach our ambitions set in our ESG Strategy.
The Top Manager representatives are responsible for cascading the ambitions and related actions through the
chain of command.
Mediq Norge are closely linked to our mother and sister companies. Mediq Norge is based in Oslo. Warehouse is
operated by our sister company Mediq Sverige based in Kungsbacka, Sweden. Several functions are organized
cross-Nordic or on global level.
Such as; Supply Chain, Sourcing, Category Management, IT, HR, Finance, Masterdata and Tender & Contract.
For Mediq Norge, our Managing Director is overall responsible for ESG in Norway.
ESG related responsibilities are found across our organization:
-Finance is responsible for setting budget according to our ESG Strategy.
-HR is responsible for ESG training programs and Equality & Diversity programs.
-Sourcing is responsible for performing Due Diligence of Suppliers. And Supplier relationship development to
improve sustainability and ethical trade.
-Supply Chain is responsible for Sustainable operations.
-Category/Product Management is responsible for Sustainable products.
-Demand Planning is responsible for analyzing demand data to optimize order predictability.
-Supply Planning is responsible for executing regular ordering to suppliers based in forcast/demand data.
-Tender & Contract is responsible for providing product information in tender bidding, to enable customers to
choose the more sustainable option.
-Marketing is responsible for publishing product information (i.e in webshop) to enable customers to choose the
more sustainable option.
-Quality/Regulatory is responsible for assisting management in anchoring policies and developing processes
related to ESG. And to support local organization in CSR issues.
Turnover in reporting year (NOK)
440 540 000
| Mediq Norge AS | 7
Number of employees
77
Is the enterprise covered by the Transparency Act?
Yes
Major changes to the enterprise since last and current reporting period
During the calendar year of 2023 there was no new acquisitions or mergers for Mediq Norge.
Contact person for the report (name and title)
Kari Solhus, Quality Manager / CSR coordinator
Email for contact person for the report
kari.solhus@mediq.com
| Mediq Norge AS | 8
Supply chain information
General description of the enterprise's sourcing model and supply chain
Mediq Norge AS is 100% owned by Mediq BV, a European market leader which proudly servers more than one
million customers.
The sourcing department, which is organized as a cross-Nordic function, as mentioned above, has a clear
description of all the activities and decision-making authorities. Across the Nordic countries, we share many of
the same suppliers.
Sourcing owns the relationship with suppliers within Mediq and negotiate prices, terms and conditions. Even if
Sourcing owns the overall agreements with supplier will there be many different points of connections between
Mediq and the supplier. Demand planning evaluates the forecasts and sets the overall demand, Supply Planning
handles all purchase orders, Finance handles supplier invoices etc.
Mediq Norge has a well established internal Code of Conduct. "Policy for responsible business conduct" can be
found on our website.
Based on our internal policy, we have developed a Supplier Code of Conduct which all suppliers have to commit
to. Ensuring that the Supplier signs and commits to our Supplier Code of Conduct is one of the responsibilities
that Sourcing has.
Number of suppliers with which the enterprise has had commercial relations in the reporting year
321
Comments
Commercial suppliers for Mediq Norge during the reporting year consists of 321. 250 of these suppliers are
considered tail end suppliers.
Type of purchasing/ suppliers relationships
Mediq Norge is not a Manufacturer and do not own any manufacturing sites.
80% of our supplies are purchased directly from the legal Manufacturer of the Medical Device. However, the legal
Manufacturer may do their manufacturing at both company owned factories or at contracted factories. Often the
legal Manufacturer provide articles manufactured from multiple factories and countries. And sometimes the
legal Manufacturer manufacture the same product on different sites. This adds complexity to the supply chain.
20% of our supplies are purchased from an Importer/Distributor.
List of first tier suppliers (producers) by country
Argentina :
1
Own or joint venture
production
0%
Direct
contracting/purchas
es
80%
Purchases through
agents/intermediary/
importers/brands
20%
Other
0%
| Mediq Norge AS | 9
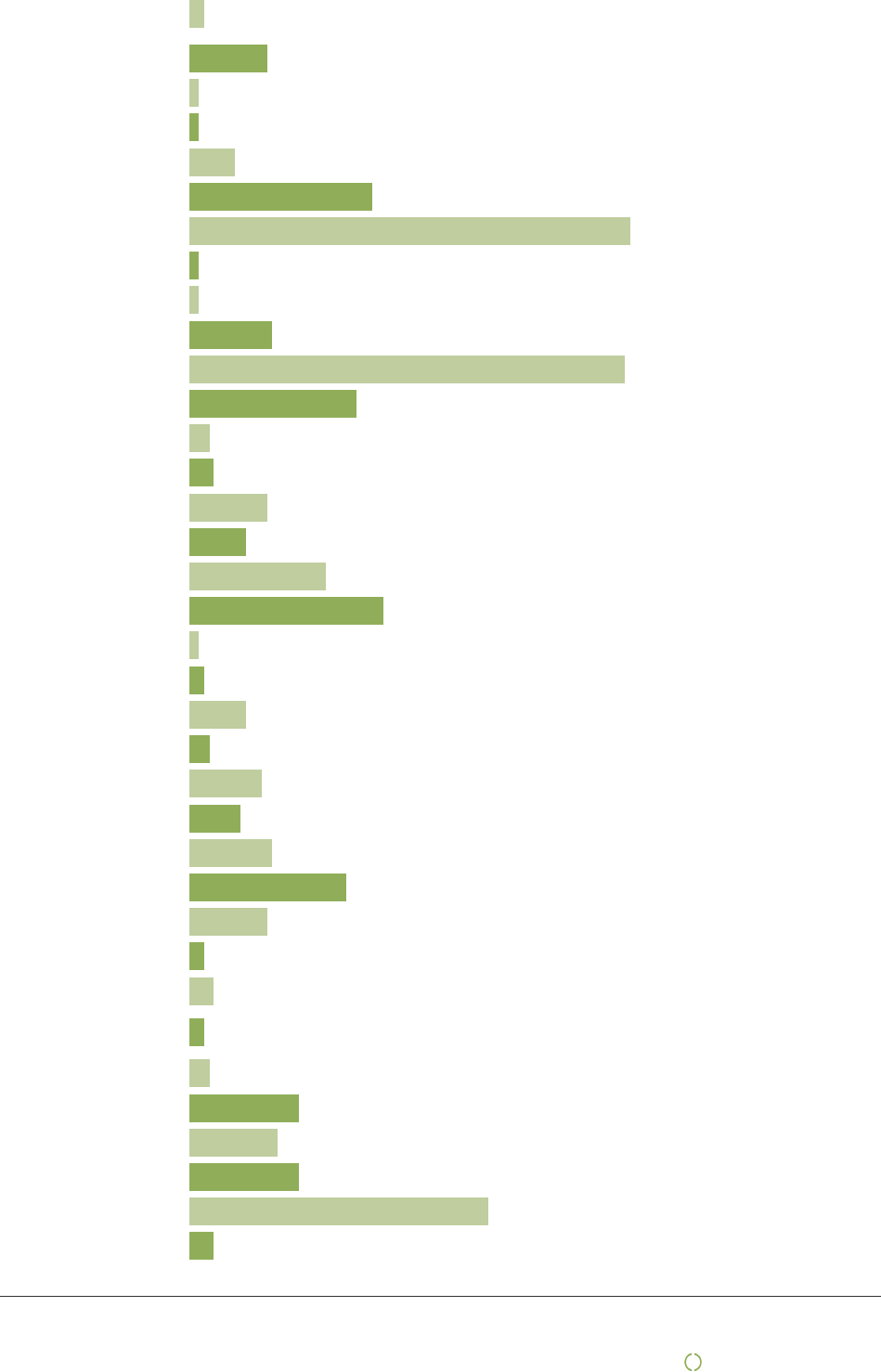
Australia :
2
Belgium :
14
Brazil :
1
Belarus :
1
Canada :
8
Switzerland :
34
China :
83
Colombia :
1
Costa Rica :
1
Czech Republic :
15
Germany :
82
Denmark :
31
Dominican Republic :
3
Estonia :
4
Spain :
14
Finland :
10
France :
25
United Kingdom :
36
Georgia :
1
Hong Kong :
2
Hungary :
10
Indonesia :
3
Ireland :
13
Israel :
9
India :
15
Italy :
29
Japan :
14
Cambodia :
2
South Korea :
4
Lithuania :
2
Latvia :
3
Mexico :
20
Malaysia :
16
Netherlands :
20
Norway :
56
New Zealand :
4
| Mediq Norge AS | 10
Philippines :
1
Pakistan :
9
Poland :
18
Puerto Rico :
1
Portugal :
6
Romania :
2
Sweden :
131
Singapore :
4
Slovenia :
1
Slovakia :
11
Thailand :
6
Tunisia :
3
Turkey :
4
Taiwan :
16
USA :
78
Uruguay :
1
Vietnam :
4
South Africa :
1
Austria :
8
Macedonia :
1
Information of Country of Origin of product is collected from the supplier as we create the different stock keeping
units (SKU) in our ERP system.
Currently Mediq Norway has about 20.000 SKU.
In 2023 Mediq Norway had transactions of about 16.000 SKU.
The legal Manufacturer may do their manufacturing at both company owned factories or at contracted factories.
Often the legal Manufacturer provide articles manufactured from multiple factories and countries. Hence, the
list above of producers is larger than the number of suppliers.
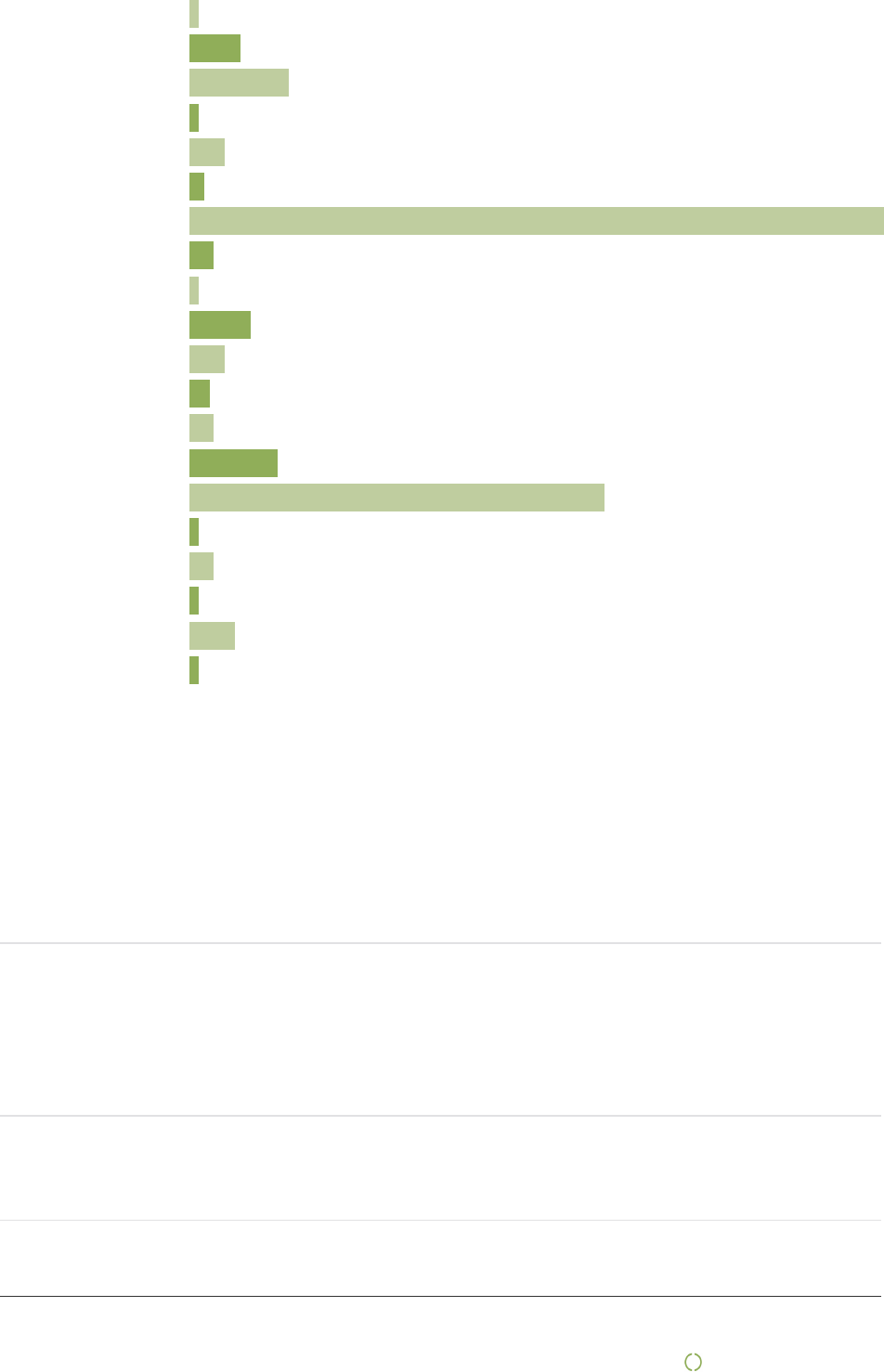
State the number of workers at first tier producers that the enterprise has an overview of, and the number of
suppliers this overview is based on:
Number of workers
111 571
Number of suppliers this overview is based on
8
| Mediq Norge AS | 11
Numbers of workers per supplier (calculated average)
13947
Comments to number of workers
The numbers of workers are based on 8 of our top 150 suppliers.
Key inputs/raw materials for products or services and associated geographies
Cotton
Global
China
India
Pakistan
Rubber
Global
Indonesia
Thailand
Vietnam
Stainless Steel
Global
United Kingdom
Indonesia
South Korea
Sweden
Plastic
Global
Mexico
USA
Aluminium Global
Mediq Norge's assortment includes ~16.000 active articles. Mediq does not at this time routinely require our
suppliers to confirm the country of origin of the Raw Materials for all of our products. This information is only
collected for selected products.
The raw materials listed here are the main raw materials for our top categories, in no particular order.
The countries and regions stated above are mainly stated due to them being large global exporters.
Is the enterprise a supplier to the public sector?
Yes
| Mediq Norge AS | 12
Goals and progress
Process goals and progress for the reporting year
1
Goal : 100% of our top 100 Nordic suppliers shall sign Mediq's Code of Conduct.
Status : 97% of top 100 Nordic suppliers have signed Mediq's Code of Conduct by end of 2023.
2
Goal :
100% of our top 20 Nordic Suppliers shall be evaluated with CSR self assessment, and a
corresponding action plan shall be created.
Status :
During 2023 Mediq changed the current risk analyses platform from Factlines to Sedex, one
common platform for whole Mediq group. Due to this fact, the completion of 2023 SAQ is delayed,
as much focus during 2023 has been on the new setup with Sedex and invite the suppliers to link
with Mediq on the Sedex platform and send out SAQ to the linked suppliers. New similar goal to
be created and followed up for 2024.
3
Goal :
Supply Chain mapping;
1. Traceability in the supply chain on our top 10 products by spend, from our High Risk list.
2. Traceability in the supply chain on our top 10 products by spend, in total.
Status : Completed.
4
Goal : 100% of our employee shall have completed our annual e-training of our Mediq Code of Conduct.
Status : 81% completed the annual e-training of our code of conduct for 2023.
| Mediq Norge AS | 13

Goal for coming years
1
100% of our employee shall have completed our annual e-training of our Mediq Code of Conduct.
2
100% of our top 100 suppliers should sign Mediq's Supplier Code of Conduct.
3
100% of top 30 Nordic suppliers (by spend) from our Mediq High Risk list should be assessed by SAQ or 3rd party
audit.
4
100% of our top 30 Nordic suppliers (by spend) sign "Confirmation on compliance with EU sanctions and
regulations regarding trade with Russia" statement.
| Mediq Norge AS | 14

1
Governance and commitment to
responsible business conduct
Embedding responsible business conduct means that the enterprise should
have strategies and plan, as well as relevant policies and guidelines for due
diligence for responsible business conduct (hereafter due diligence) which
are adopted by management. These should comprise the enterprise’s own
operations, its supply chain and other business relationships. Effective
management systems for implementation are key to success, and due
diligence should be an integrated element in enterprise operations. Clear
expectations from senior management are crucial, as well as clearly assigned
responsibilities within the enterprise, for the implementation of the steps in
the due diligence process. Those involved need to know how to proceed.
Transparency about commitments the enterprise has for itself, challenges
they are facing, and how these are managed is fundamental

1.A Policy* for own enterprise
1.A.1 Link to publicly accessible policy for own enterprise
https://mediqnorge.no/om-oss/csr
1.A.2 What does the enterprise say publicly about its commitments to respect people, animals, society, the
environment and climate?
Mediq has established a set of Mediq Code of Conduct (https://mediqnorge.no/om-oss/code-of-conduct) which
all companies in the Mediq Group need to comply according to. This document highlights Mediq`s core values
and mission in addition to describing Mediq's expectations in regards to:
• People and Environment
- Safe Workplace
- Workplace violence
- Alcohol and drug-free workplace
- Human Rights (with listing of relevant conventions and guidelines)
- Anti-Discrimination
- Anti-Harassment
- Diversity, Equity & Inclusion
- Environment
- Animal Welfare
• Business Integrity & Fairness
- Conflicts of interest
- Anti-Kickback, Bribery and Corruption
- Dealing with government officials, Healthcare professionals, Healthcare insurers and other payors
- Quality
- Exports and anti-money laundering
- Antitrust and competition laws
- Gifts, entertainment, hospitality and donations
• Safeguarding of company assets
- User of company resources & property
- IT use guidelines
- Fraud
- Privacy & data protection
• Instruction of reporting suspected breach of conduct on anonymous reporting hotline (www.speakup
feedback.eu)
Based on our Mediq Code of Conduct, Mediq has developed a Supplier Code of Conduct
(https://mediqnorge.no/om-oss/csr) that all companies in the Mediq Group use towards our suppliers.
The Supplier Code of Conduct requires that all our suppliers commits to the same principles throughout the
whole value chain. The ethical guidelines are designed to ensure that the production of our goods complies with
human- and labor rights.
Mediq Norway are ISO14001 certified. Our Environmental Management system support Mediq Norway to
minimize environmental impact and ensure operations in compliance with local laws and regulations. This
allows Mediq Norway to continuously measure and improve the way our business affects the environment. Our
certificates are published on our website.
Mediq is committed to upholding ethical labor practices and procedures across all of its locations. Our
responsibility in this area includes creating awareness and understanding of human rights, employment, and
labor practices. By incorporating these principles into strategies, policies, and procedures, and living out our
values, Mediq will uphold our responsibilities to people, environment, and set the stage for our long-term
success.
Mediq supports and respects the protection of internationally proclaimed human rights, and we strive to ensure
that we are not complicit in human rights abuses. We also uphold the freedom of association and the effective
recognition of the right to collective bargaining, the elimination of all forms of forced and compulsory labor, and
| Mediq Norge AS | 16
the effective abolition of child labor.
Our principles regarding the quality, environment and ethical labor practices are founded on UN and
International Labor Organization conventions as amended or restated from time to time.
Mediq Norge uses our website to communicate towards our external stakeholders how we commit to our work
doing our due diligence in our supply chain. Our "Policy for Responsible Business Conduct" and a description of
how Mediq work with Corporate Social Responsibility towards our suppliers are published on our website.
In 2023 Mediq relaunched our ESG Strategy. You can read more in pt 1.C.1 and on our website.
(https://mediqnorge.no/om-oss/csr)
1.A.3 How has the policy/commitment been developed and how is it embedded in the enterprise?
The sender of our Mediq Code of Conduct is the CEO of the overall Mediq Group.
The policy is on the agenda from board meetings down through sales meetings, purchasing meetings, and
supplier contract.
Mediq Code of Conduct is developed by Top Management together with our Group ESG Committee. Local
country ESG coordinators gives their input to the Group ESG Committee.
Our Mediq Code of Conduct on group level are currently not describing policy for Regular Employment (ILO
Convention No 95, 158, 175, 177 and 181). This is only stated in our Group Supplier Code of Conduct. Hence, Mediq
Norge has a national policy document "Policy for responsible business conduct" to include this topic
(https://mediqnorge.no/om-oss/csr).
This policy document is based on resources from Etisk Handel Norge, approved by the board of Mediq Norge and
signed by Managing Director of Mediq Norge.
The Mediq Code of Conduct is part of our mandatory annual e-training module for all employees in Mediq
Group.
The onboarding process of new employees at Mediq Norge also include face to face training on ESG topics with
the local ESG coordinator.
Also, the company's intranet is used to communicate with all employees about the work on ethical trade and
risks in the value chain. Including communication regarding our member reporting to the Ethical Trade
Initiative in Norway, as well as the risks and issues we see in the markets we operate in.
Our Mediq Code of Conduct applies to all employees, officers, and directors of Mediq and governs all our
decisions and actions, whether in our offices, warehouses, in the boardroom, at customer or supplier premises or
when providing care to our patients. This Code is at the center of everything we do. It reinforces our Core Values.
We require that all our suppliers commit to our Supplier CoC, to ensure that the same principles are followed
throughout the value chain.
Lastly, Mediq Norge has established internal procedures related to ESG in our Management System.
Mediq Norge is certified according to ISO9001 and ISO14001.
| Mediq Norge AS | 17
1.B Organisation and internal communication
1.B.1 How is the due diligence work organised within the enterprise, embedded in internal guidelines and routines,
and why?
The ESG policy and strategy are decided by our CEO for Mediq Group. Our Managing Director for Norway is
overall responsible for ESG within Norway.
Within Mediq the responsibility for performing Supplier Due Diligence is placed to our Sourcing department.
The reporting lines within Sourcing are:
• CEO
• Chief Commercial Officer
• Vice President Sourcing
• Nordic Sourcing Director
• Nordic Sourcing Manager
• Nordic Sourcing Specialist
Vice President Sourcing is part of our Group ESG Committee (as previously described in pt “Description of
Enterprice Structure"). Hence, the Vice President Sourcing have been part of developing our ESG Strategy. The
role is responsible for implementing actions throughout the reporting lines for the different countries to ensure
we will meet our ambitions set out in our ESG Strategy. A Road Map 2030 are currently under development.
Personal incentives will be set to ensure adherence to the Road Map 2030.
The Nordic countries in Mediq have many shared functions, such as Sourcing. The Nordic countries share many
of the same suppliers. Nordic Sourcing Specialist is responsible for the practical performance of the Supplier Due
Diligence for the Nordic countries, by the use of the external company Sedex.
During 2023 the different Mediq countries have been harmonizing our Ways of Working. All Mediq countries
now use Sedex as partner for Due Diligence.
Sedex (Supplier Ethical Data Exchange) is a global non-profit organization headquartered in London, UK. It
operates one of the world's largest collaborative platforms for sharing responsible sourcing data on supply
chains. Sedex enables businesses to efficiently manage and report on ethical and responsible practices within
their supply chains, addressing issues such as labor rights, health and safety, environmental impact, and
business ethics. Through its platform, Sedex facilitates transparency and collaboration among companies,
suppliers, and stakeholders to drive improvements in supply chain sustainability.
Sourcing Specialist prepares an annual summary report the performed Supplier Due Diligence. In the light of the
upcoming Road Map 2030, it is likely that the internal reporting routines will be changed from 2024.
The annual report of Due Diligence for Responsible Business Conduct are shared with and signed by the board of
Directors.
Group ESG Manager assist Top Management in anchoring policies, developing processes and tools related to
ESG, and to support all business units (Mediq Countries) in ESG matters.
The local country ESG Coordinators assist local Management, ensure local routines, coordinate reporting and
internal communication, as well as promoting local requirements up to Group level.
| Mediq Norge AS | 18
1.B.2 How is the significance of the enterprise's due diligence work defined and clarified for the employees through
their job description (or the like), work tasks and incentive structures?
Mediq has set up an e-training module "Mediq's Ethical Guideline" in our digital tool "Mediq Academy".
The e-training includes a video presentation from our CEO, an explanation of why we need a Code of Conduct
and the actual Mediq Code of Conduct. The theoretical training is followed by a test. The e-training module is
mandatory for all employees on all levels and must be performed annually.
On local level, the onboarding process at Mediq Norge include a section of ESG training conducted by the local
ESG coordinator in Mediq Norge. This training is face-to-face training that includes more details about local
Norwegian requirements and stakeholder expectations.
Personnel with specific tasks related to Due Diligence have Job Descriptions that describes these responsibilities
and tasks. This applies for i.e Nordic Sourcing Specialist and local ESG Coordinator.
Individual training programs are set up for new employees within these roles. The training program includes
process descriptions and procedures in our Management System, as well as materials and webinars available at
Etisk Handel Norge.
Personal incentives were not implemented in 2023, but are part of the plan for Road Map 2030.
1.B.3 How does the enterprise make sure employees have adequate competence to work on due diligence for
responsible business conduct?
Mediq ensure suitable competence for performance of Due Diligence for responsible business conduct by setting
up individual training programs for our employees. The training program includes:
• Relevant processes and procedures in Mediq Management System
• Etisk Handel Norge resources, courses and webinars
• Direct training from Sedex
• Sharing of best practices in Supplier & Customer meetings
• Sharing of best practice internally
• Negotiation courses
• Leadership programs
• Higher educations
| Mediq Norge AS | 19

1.C. Plans and resources
1.C.1 How are the enterprise's commitments to respect people, animals, society and the environment embedded in
strategies and action plans?
Mediq Norge AS strives towards responsible business conduct that respects people, society and the environment.
Mediq considers responsible business conduct to be a prerequisite for sustainable development, meaning that
today’s generation get their needs covered without compromising the ability of future generations to meet their
own needs.
This is in line with our Core Values: Caring Hearth, Customer Drive and Champion Spirit .
During 2023 Mediq have relaunched our ESG strategy. As Mediq is continuously evolving, our strategies and
frameworks must do, too.
We have transitioned from a CSR-focused approach to a more encompassing ESG model.
For those unfamiliar, while Corporate Social Responsibility (CSR) has traditionally emphasized ethical
operations and sustainability initiatives, the Environmental, Social, and Governance (ESG) framework takes a
broader view. ESG not only addresses the negative impacts, but focuses on minimizing those impacts and
redirecting them toward creating positive outcomes.
Our dedicated ESG Committee refined and expanded our ESG strategy during 2023. And the outcome of this
work is a comprehensive and ambitious approach. This enhanced strategy now encapsulates our entire value
chain—from the production phase of our products to their eventual disposal by end-users.
While developing the new ESG strategy, we've examined the positive and negative environmental and social
footprints we leave behind. Now, our strategy is structured around four key pillars:
• Our products
We deliver products with minimal environmental impact – keeping circularity as our guiding principle – that
are ethically produced.
• Our services
We provide services and solutions to enrich the quality of life of patients and people working in healthcare and
support the sustainability transition in healthcare.
• Our operations
We operate minimizing waste, use of packaging material, emission in transport and energy use in buildings.
Keeping circularity as our guiding principle.
• Our people
We develop and empower engaged, healthy and diverse people.
You can read the full description of Mediq’s ESG Strategy, with description of the four key pillars, focus areas,
ambitions and corresponding UN sustainable development Goal, on our webpage.
https://mediqnorge.no/om-oss/csr
https://mediq.com/about-us/environmental-social-governance
Our ESG Committee are currently working on a Road Map against 2030, in order to reach our Ambitions that are
outlines in the ESG strategy.
For Mediq Norge the definition of our new ESG strategy and setting a Road Map against 2030 is essential for
being a player in the public tender biddings and to comply to the Norwegian Transparency Act.
See further description of resources and organizational set up in “Description of enterprise structure” and 1.B.1.
| Mediq Norge AS | 20

1.C.2 How is the enterprise’s strategies and action plans to work towards being responsible and sustainable followed
up by senior management and the board?
For Mediq Norge, it is the local management team that have been responsible for following up on the work with
the different support functions in the Nordic countries, with regards to responsible business conduct and
sustainability, with the Managing Director being the overall responsible for setting the agenda for Mediq Norge
by making sure that:
• The achievement of the company's aims for the given year
• The company's strategy and the risks inherent in its business activities
• The compliance with legislation and regulations
I.e during 2023, Head of Sourcing have reported monthly to Management Team in Mediq Norge on status and
progress, including CSR related issues.
By the end of 2023 Mediq Group have taken large steps in anchoring the responsibility of the ESG strategy in the
functional reporting lines up to Top Management representatives, for the key areas PRODUCTS, SERVICES,
OPERATIONS and OUR PEOPLE.
In 2024 personal goals related to these key areas in our ESG strategy will be developed and followed up through
this functional reporting line, to promote achievements within our ESG ambitions.
See “Description of enterprise structure”.
| Mediq Norge AS | 21

1.D Partnerships and collaboration with business relationships, suppliers in
particular
1.D.1 How does the enterprise emphasise the importance of responsible and sustainable business conduct in its
business relationships, particularly in the supply chain?
As part of the process that our Nordic Sourcing department follows when a contract with a new supplier are to be
entered, Mediq require that our Supplier Code of Conduct read, understood and signed. The Supplier Code of
Conduct is a mandatory attachment to the commercial agreement. The Supplier CoC require that our suppliers
commit and adhere to the requirements, that they have the training and tools to do so, and that they shall be able
to document their efforts to secure compliance with the local laws and our Supplier Code of Conduct at our
request. This also applies to any sub-supplier. Mediq may terminate the relationship with any supplier, third
party representative or other business partners that fails to meet the standards in this Code after a reasonable
period of time for remedying a breach.
Other attachments to our commercial agreement include “Guidelines for environmentally adapted choice of
products”.
Our minimum requirements to our suppliers include that they shall sign our Mediq Supplier Code of Conduct, or
provide us with an equal statement. This commits the Supplier to actively communicate the content of the
Supplier Code of Conduct to their workers as well as to their suppliers. The Suppliers must at minimum require
that its suppliers (our second tier supplier) acknowledge and implement a corresponding Code of Conduct
requirements. The Supplier Code of Conduct also requires the Supplier to provide Mediq with information by
responding to supplementary questionnaire, as well as allowing Mediq or 3rd party to perform audits. (As listed
in pt 6 in Supplier Code of Conduct).
All new manufacturing partners for Mediq Own Brand products are subject to a supplier visit and evaluation with
a multifunctional team.
As described in pt 1.B.1, Mediq changed our partner from Factlines to Sedex in 2023. When collaborating with
Factlines, a risk score method was used as a tool for screening our suppliers. During 2024 we will develop our
collaboration with Sedex and define routines and responsibility regarding screening of suppliers within the
Sedex system. Sedex provides us with digital solutions and services, to monitor how closely our suppliers comply
to the Mediq Supplier Code of Conduct and to identify areas for follow-up. Prioritized concerns are followed up
with the supplier by our Sourcing department. The results from the CSR profile report is discussed, and sourcing
department collaborates with the supplier with any Corrective Action Preventive Action that may result from
this.
To communicate Mediq policies, Mediq Norge has uploaded "Policy for responsible business conduct" and
"Responsible Sourcing Program" to our website: https://mediqnorge.no/om-oss/csr.
Mediq acknowledge that our purchase practices influence the suppliers ability to comply to our CoC. It is
important for our suppliers to have predictability and Mediq strive to give good and accurate information of
forecasts to our suppliers. To ensure this our Demand Management team analyze demand data to optimize order
predictability and regularity. Supply Planning is responsible for executing regular orders based on well-founded
demand data.
Indicator
| Mediq Norge AS | 22

% of signing of Mediq Code of Conduct for our top 100 Nordic suppliers shall sign Mediq's Code of Conduct.
2023
97%
2022
95%
2021
88%
| Mediq Norge AS | 23

1.E Experiences and changes
1.E.1 What experiences have the enterprise encountered during the reporting period concerning responsible business
conduct, and what has changed as a result of this?
As described in EHN report for 2022, Mediq experienced a clear reduction in response rate to the 2022 SAQs. We
saw the need to do changes to improve the tools to perform our Due Diligence. This resulted in a change for the
entire Mediq Group by transferring to Sedex, as our Due Diligence partner in 2023. By using Sedex we now have
access to a pool of audit reports to supplement the SAQ data.
During 2023 we have focused on implementing the Sedex system, identifying suppliers to be assessed, sending
SAQs and collecting audit reports. However, due to the transfer Mediq was not able to complete the Due
Dilligence assessments of the prioritized suppliers for 2023. The process will continue into 2024.
As previously described in 1.C.1 Mediq have seen the need to shift focus and strategy towards a more
comprehensive and integrated approach to sustainable business practices. As a consequence Mediq have
relaunched our ESG strategy to a more encompassing ESG model.
| Mediq Norge AS | 24

2
Defining the focus for reporting
Identify and assess the
enterprise's impact on people,
animals, society and the
environment
“Identify and assess” is about identifying the enterprises's risk for, and actual
negative impact on, people, animals, society and the environment, including
in the supply chain and through business relationships. As a first step the
enterprise should get an overall risk picture, before subsequently prioritising
further mapping and measures where the risk of negative impact is the
greatest, i.e. salient issues. The enterprises's involvement in the negative
impact on people, animals, society and the environment is central to
determine which measures the enterprise should implement in the next step
of the due diligence model. Involvement of stakeholders, especially those
affected, is central when assessing risks. It is also important to consult with
stakeholders when implementing measures to manage the negative impact.
2.A Mapping and prioritising
PRIORITISED ACTUAL OR POTENTIAL NEGATIVE IMPACT ON PEOPLE, ANIMALS, SOCIETY, AND
THE ENVIRONMENT
Prioritising one or more risk areas on the basis of severity does not mean that some risks are more important than
others, or that the company should not take action on other risks, but that risks with the greatest negative impact
are prioritised first. Mapping and prioritisation are a continuous process.
2.A.1 List the enterprises's actual negative impacts and/or prioritized significant risks of negative impact/harm on
people, animals, society and the environment. Take note that the prioritized risk that you list in the table below will be
exported to step 3 of this report, where you will be asked to answer how you work with stopping, preventing, or
reducing the negative impact.
Salient issue Related topic Geography
Breach of labor- and human rights for
employees at suppliers on Mediq High Risk List.
(Medical supplies in our categories Personal
protection, Surgical & OR and Wound care &
compressions / Medical supplies from selected
countries. )
Occupational Health and
safety
Wages
Working hours
Regular employment
China
Malaysia
Environmental impact from Transport
Emissions, Energy use in buildings, Packaging
Materials and Waste.
Environment
Emission
Greenhouse gas emission
Energy
Waste
Use of materials
Global
Norway
Sweden
Conflict areas / War zones
Israel
Russia
Mediq Norge is an importer/distributor of Medical Devices. We do not have our own production. Our suppliers
are typically a legal Manufacturer that either does their own manufacturing, or have contracted the
manufacturing to another partner. In this way Mediq Norge are directly linked to the manufacturing either
through one or more entities.
Among the sources for risk assessment that are described in pt 2.A.2, we have found that the high risk product
groups medical consumables/equipment and textiles, work clothes and footwear from DFØ´s "The high-risk list"
at Anskaffelser.no represent our portfolio very well.
From this we have reviewed our different product categories and chosen to prioritize suppliers of the following
Mediq Product Categories:
- Surgical & OR, and sub-category Instruments
- Wound care & compression, and sub-category Compression
- Personal Protection, and sub-categories Examination Gloves & Personal Protection
| Mediq Norge AS | 26

Mediq has also prioritized to focus environmental impact from Transport Emissions, Energy use in buildings,
Packaging Materials and Waste.
JUSTIFICATION FOR THE PRIORITISATION OF RISKS OF NEGATIVE IMPACT ON PEOPLE,
ANIMALS, SOCIETY, AND THE ENVIRONMENT
2.A.2 Describe: a) the enterprise's routines for mapping and identifying risk and show how the negative impact was
identified and prioritised in this period: b) eventual aspects of the enterprise that have not been covered in this report
(product groups, own products, departments etc.) and why you not chose to prioritize these in the continued work: c)
how information was gathered, what sources were used, and which stakeholders have been involved/consulted: d)
whether you have identified areas where information is lacking in order to get an overview, and how you are planning
to proceed to collect more information/handle this.
As mentioned earlier in this report, Mediq Own Brand products are manufactured by our sister company,
Medeco, in the Mediq Group. Medeco is the legal Manufacturer of these products and the production is
outsourced to strategic manufacturing partners. Medeco follows the same framework as the rest of Mediq Group,
based on our common Code of Conduct.
In order to have close control of the contracted manufacturers Medeco are member of Amfori BSCI, a leading
global business association for open and sustainable trade.
Amfori creates a platform which actively monitors and shares supply chain information to ensure transparent
and sustainable trade. They provide a network of independent, accredited audit companies that monitor and
evaluate factories according to the eleven BSCI principles. When not available via Amfori, Medeco audits
factories with an independent third party. Medeco performs annual due diligence on the social performance and
improvement planning of the manufacturing partners. The outcome is monitored and tracked by the
management, and when required, supplemental actions are initiated. The information is shared with our Nordic
Sourcing department, and in this way part of Responsible Sourcing program for the Nordics.
In addition to the Mediq Own Brand product provided by Medeco, Mediq Norge has about 300+ external
suppliers. Some of them are the legal Manufacturer, others are Importers or Distributors. The legal Manufactures
may have multiple production sites. Some of the production sites might be their own, while others are contracted
production sites. This results in a very large amount of productions sites. For this reason we need to prioritize
and focus our work.
For our external suppliers Mediq Nordics has chosen to do a screening of the suppliers to focus based on type of
product. As mentioned in pt 2.A.1 Mediq has found that the high risk product groups medical
consumables/equipment and textiles, work clothes and footwear from DFØ´s "The high-risk list" at
Anskaffelser.no represent our portfolio very well.
Based on this, Mediq has identified our corresponding product categories:
- Surgical & OR & sub-category Instruments
- Wound care & compression & sub-category Compression
- Personal Protection & sub-categories Examination Gloves & Personal Protection
With information about risk countries, provided by Sedex and the high risk product groups identified above, we
have created our “Mediq High Risk List”. All suppliers that deliver products within these high risk countries and
high risk product categories are flagged in this list. Medeco is being one of them. For our annual due diligence
the suppliers on the high risk list was prioritized according to spend.
In 2023 this resulted in 30 selected high risk suppliers covering 17% of the Nordic spend.
Sedex invites Mediq suppliers to link with Mediq on the Sedex platform and once they are linked with Mediq,
they are asked to fill in a self assessment questionnaire (SAQ). When the SAQ is completed the supplier is
assessed by Sedex and get a site characteristics risk score based on the SAQ answers & the overall inherent risk.
Based on the risk score and Mediq internal policy/requirements Sedex can recommend which sites are most
suitable for audits.
When a high risk site is audited, the auditor will upload a corrective action plan report (CAPR) to the Sedex
platform containing any non-compliances found during the audit and a recommended time frame for corrective
action.
| Mediq Norge AS | 27

Mediq has an Ethical Coordinator at Sedex, who will monitor these audits and follow-up suppliers to make sure
actions are taken to close the non-compliances.
Sedex risk assessment steps:
Self-Assessment Questionnaire (SAQ) - Collect data
Suppliers complete the SAQ about their business practices and upload this into Sedex platform.
The SAQ covers the following topics:
-Workplace Impact
-Management Systems for Code Implementation
-Freely Chosen Employment
-Freedom of Association
-Health and Safety
-Living Accomodation
-Children and Young Workers
-Wages
-Working Hours
-Discrimination
-Regular Employment
-Discipline and Grieviance
-Environment
-Business Ethics
Risk Assessment (Radar) - Assess data & risk
Sedex assesses suppliers´ information using Radar.
Radar is Sedex’s comprehensive risk assessment and analysis tool. Members use Radar to understand what the
most likely issues in their supply chains will be, even at the earliest stages of risk assessment.
Radar uses hundreds of data sources to produce scores, on a scale of 0 – 10.0, across 14 issue areas, including:
- Forced labour
- Freedom of association
- Gender inequality
- Health, safety and hygiene
- Wages
- Waste and pollution
- Water stress
These scores act as an indication for the level of risk within different countries and industries. The higher the
score, the higher the risk.
For example, in the “Working Hours” issue area, a score of 10 would indicate that workers are at the highest risk
of working excessive hours.
Radar also incorporates data on a business’s suppliers, where this is available from audits and the self-
assessment questionnaire, to produce unique risk scores for individual sites in a supply chain.
Radar comprises hundreds of data points from independent, authoritative sources on human rights and
environmental risks in supply chains.
The sources for risk scores used is listed below*.
Sedex Members Ethical Trade Audit (SMETA) - Assess data & risk, analyse & report
Higher risk suppliers will be requested to undergo a SMETA audit by a third party.
Reporting tools (Analytics) - Analyse & report data
Analyse and report on the data using Sedex analytics.
E-Learning & Training - Learn how to implement improvements
Use Sedex e-learning and guidance to resolve audit non-compliances and improve social, ethical and
environmental business practices.
Follow up of SAQs:
Before transfering to Sedex the Nordic Sourcing department prioritized and prepared a list of follow-up activities
| Mediq Norge AS | 28

in a Corrective Action Plan (CAP) based on information given from our previous partner Factlines.
The findings and corresponding CAPs were communicated to and followed up with the Suppliers by Mediq, for
further dialogue to solve issues.
Information on prioritized risks, results and assessment of collected information was shared with Category- and
Product Management, so findings could be used to further work with the product selection. This information
was also used towards our customers either when discussing in direct meetings where CSR was a topic, or in the
surveys that we received from them.
For the CAP list created in 2022 we had 80 actions to follow up during 2023. A handful is still remaining and will
be followed up into 2024.
As mentioned in 1.E.1 the Due Diligence assessment for 2023 was not completed by the end of the year, due to the
work related to transfer to Sedex. By the end of the year, 5 of the approx. 30 selected suppliers have completed
the SAQ. The preliminary results show overall rating by Sedex for these suppliers as low risk, and no severe risk
was identified among the suppliers answering the survey.
Due to the transfer to the new partner Sedex the way of working with Corrective Actions is not yet defined. Most
likely the follow-up of corrective actions will be supported by Sedex for suppliers with a high risk score.
Our new partner Sedex initially focuses primarily on introducing new suppliers to the platform and getting them
connected to Mediq and getting them to complete the SAQ. Next step is performing complete evaluations
according to prioritization by Mediq, and to recommend which to undergo a 3rd party audit. When audits are
made Sedex will look in to the CAP reports that are automatically sent to the Sedex platform and Sedex will
follow up the suppliers with highest risk to make sure they take agreed actions on the non conformities.
In addition to annual surveys, we act on information on risks that are brought to our intention by other means.
This could be from customers, business partners, news articles and peer reviewed papers.
Areas not covered in this report:
In regards to products we assess as low risk, we have not performed any follow up actions in 2023.
Nor any actions towards our service providers, that are based in Norway, Sweden or Northern Europe, as we
consider these countries to be low risk. The extent of activities for these suppliers are limited to getting our
Supplier Code of Conduct signed.
Sources*:
• High risk products - The Norwegian Agency for Public and Financial Management (DFØ)
https://www.anskaffelser.no/public-procurement/socially-responsible-publicprocurement/information-about-
high-risk-products
Topic - Indicator - Source (as used by Sedex):
• Gender - Gender inequality - Index United Nations Development Programme (2022)
• Gender - Global Gender Gap - World Economic Forum (2022)
• Gender - Women, Business and the Law Custom subset - World Bank (2023)
• Forced Labour - Forced Labour Index - Ergon Associates (2022)
• Freedom of Association and Collective Bargaining - ITUC Global Rights Index - The International Trade Union
Confederation (ITUC) (2023)
• Health, safety & hygiene - Environmental Performance Index (EPI): Sanitation & Drinking Water - Yale
University (2022)
• Health, safety & hygiene - Global Health Security (GHS) Index: Social resilience - Nuclear Threat Initiative (NTI),
the Johns Hopkins Center for Health Security (JHU), The Economist Intelligence Unit (EIU) (2022)
• Health, safety & hygiene - The Notre Dame-Global Adaptation Index (ND-GAIN) Country Index - University of
Notre Dame (2021)
• Children & Young Workers - Children's Rights in the Workplace - Global Child Forum and UNICEF (2018)
• Wages - 2022 Country Reports on Human Rights Practices - US Bureau of Democracy, Human Rights and Labor
(2023)
• Wages - Poverty headcount ratio at $6.85 a day (2017 PPP) (% of population) - World Bank (2022)
• Working Hours - Mean weekly working hours actually worked per employee - ILO (2023)
• Discrimination - Group Grievance - Fund for Peace (2023)
• Discrimination - Global Slavery Index vulnerability Model: Disenfranchised groups - Walk Free Foundation
(2023)
| Mediq Norge AS | 29

• Discrimination - Freedom in the World - Freedom House (2023)
• Regular employment - Wage and salaried workers, total (% of total employment) - World Development
Indicators World Bank / ILO (2023)
• Business Ethics - Corruption Perception Index - Transparency International (2023)
• Biodiversity - Environmental Performance Index (EPI): Biodiversity & Habitat - Yale University (2022)
• Biodiversity - Environmental Performance Index (EPI): Ecosystems services - Yale University (2022)
• Energy & Emissions - Environmental Performance Index (EPI): Climate Change - Yale University (2022)
• Water - Water Stress Index - World Resources Institute (2019)
• Waste and pollution - Environmental Performance Index (EPI): APE – pollution emissions - Yale University
(2022)
• Waste and pollution - Environmental Performance Index (EPI): WMG – Controlled solid waste - Yale University
(2022)
ADDITIONAL SEVERE IMPACTS
2.A.3 Describe any other negative impacts on people, animals, society and the environment that were identified in the
mapping of the enterprise, supply chain or other business relationships during the reporting period and how these
have been handled.
As described in our EHN 2022 report, Mediq took actions when Russia invaded Ukraine to ensure that we did not
have any products in our assortment with Russian connections. A small number of items where identified, but
the relevant suppliers immediately took actions to end their collaboration with their Russian connections. I.e by
moving production to other factories.
As the sanctions against Russia has evolved, Mediq have during 2023 requested our suppliers to sign a
"Confirmation on compliance with EU sanctions and regulations regarding trade with Russia".
Concerning the ongoing Israel/Palestine conflict Mediq has assessed our suppliers. The suppliers and
manufacturing sites in Israel was investigated to see if they are based within the conflict areas of Golan heights,
West Bank or Gaza. None of the suppliers are related to these areas. We will continue to follow the situation
closely.
| Mediq Norge AS | 30

3
Management of salient issues
Cease, prevent or mitigate
negative impacts
“Cease, prevent and mitigate” is about managing findings from the risk
assessment in a good way. The most salient negative impact on people,
animals, society and the environment should be prioritised first. This does
not mean that other risks are insignificant or that they are not handled. The
way the enterprise is involved in the negative impact is key to taking the
appropriate action. Negative impact that the enterprise causes or contributes
to must cease, be prevented and be reduced. To address negative impact
directly linked to the enterprise, e.g. in the supply chain, the business must
use its leverage to in¬fluence the entity causing the negative impact to cease,
prevent or mitigate it. This involves developing and implementing plans and
routines to manage risk and may require changes to the enterprise's own
policy documents and management systems. Effective management of the
negative impact on people, animals, society, and the environment is a major
contribution to the achievement of the Sustainable Development Goals
(SDGs).
3. A Cease, prevent or mitigate
3.A.1 For each salient risk, add a goal, progress status and describe the measures you have implemented to handle
the enterprise’s prioritized negative impact on people, animals, society, and the environment
Salient issue
Breach of labor- and human rights for employees at suppliers on
Mediq High Risk List.
(Medical supplies in our categories Personal protection, Surgical &
OR and Wound care & compressions / Medical supplies from
selected countries. )
Goal :
Reduce negative impact on labor- and human rights.
Status :
Addressing the prioritized findings from the Supplier Assessment
Questionnaire to the suppliers.
Goals in reporting year :
Ensure that our requirements in our Code of Conduct is understood and
appropriately implemented by our suppliers.
| Mediq Norge AS | 32

Describe already implemented or planned measures to cease, prevent or mitigate negative impacts and
reasoning behind the selected measures :
Mediq strive to impact the practice of our suppliers through open dialogue. In this way we have
during 2022 and 2023 closed the majority of the CAP list set up in 2022.
Typical action points towards our suppliers was; New routine implementation,
Clarification/Verification of answer and signing Supplier Code of Conduct.
Examples of new routines that required implementing at supplier:
-Ethical Guidelines/Code of Conduct for their suppliers
-Systems/routines for follow-up of the supply chain
-Anti-bribery and Anti-corruption policy
-Whistleblower procedures
Due to the transfer from Factlines to Sedex we have no overall risk rating available yet from the SAQ
´s initiated in 2023, as the main focus from Sedex now is to link suppliers with Mediq on the platform
and make sure they will complete the SAQ. The next step for Sedex is to assess the SAQ´s and take
further actions if needed, such as audits and corrective action plans.
For 5 of our suppliers on the high risk list that have completed their SAQ´s, we can see that the
combined overall risk category were low.
Through audit reports we have been able to look further into the following examples:
For a manufacturing site for gloves in Malaysia, audit revealed three Non Conformities related to
Health and Safety; Lacking fitness certificate for passenger lift. Fire- and electricity hazard was not
evaluated for the dormitory.
For two manufacturing sites for Covid tests in China, audits revealed the following examples of Non
Conformities: Excessive use of overtime. Inappropiate level of insurance for employees. Missing local
language on chemical safety labels. No secondary containment of chemicals.
Findings from ongoing SAQs and available audit reports will be prioritized and followed up in
collaboration with Sedex.
Describe actual or expected results of measures mentioned above, as well as goals and activities for the
coming reporting year :
As Mediq are now in Onboarding phase of using Sedex, we expect that we will develop and improve
the way we work and use the tools to get better data about our Supply chain, through both SAQs and
Audit reports. More detailed knowledge will give us a better platform to drive the dialogue for
improvement with our suppliers.
| Mediq Norge AS | 33
Salient issue
Environmental impact from Transport Emissions, Energy use in
buildings, Packaging Materials and Waste.
Goal :
To be climate gas neutral and circular company.
Status :
Group KPIs and reporting lines have been set for various KPIs related to
environment.
Goals in reporting year :
Continue reporting on our established KPIs. Evaluate trend and effect of
various project to reach set targets.
Examples of KPIs:
- We aim to decrease residual waste with a year-by-year reduction of 5%.
- We aim to decrease electricity consumption with a year-by-
year reduction of 2%
- We aim to decrease packaging carton consumption with a year-by-year
reduction of 2%
- We aim to decrease packaging plastic consumption with a year-by-year
reduction of 2%
- We aim to decrease CO2 emission per kg sent by pallet with a year-by-year
reduction of 5%
Describe already implemented or planned measures to cease, prevent or mitigate negative impacts and
reasoning behind the selected measures :
Mediq has implemented several actions to reduce transport emission and amount of packaging
materials used in our warehouse. This includes working close with our transport partner to optimize
for emission neutral transport. Efforts to reduce transport of air include optimizing transport box
size, based by improved calculation of order volume and using automatic packaging process that cut
the height of box sides according to content level.
Efforts to reduce use of plastic include using carton instead of plastic as fill material and using
cellulose material for delivery note poach. Mediq use transport boxes of unbleached fibers with high
degree of recycled materials, that are FSC certified and labelled with recycling information. In 2023
we changing our machines for plastic wrapping that stretches the plastic more efficiently. We only
use transparent (not black) plastic for wrapping of pallets to ensure recyclability.
Mediq encourage customers to place orders in whole transport boxes, to avoid repacking into new
packaging materials. We help customers in different ways to avoid rush orders outside of predefined
delivery dates.
As mentioned in 1.C.1, Mediq has set a new ESG strategy and are currently working on a Road Map
against 2030 to reach our ambitions.
| Mediq Norge AS | 34
Describe actual or expected results of measures mentioned above, as well as goals and activities for the
coming reporting year :
In our Warehouse the amount of residual waste was reduced by 55% from 2022 to 2023.
Reduction of CO2 emission pr kg sent by pallet from 2022 to 2023 by 3,5%.
As Mediq Norge and Mediq Sverige share Warehouse, it is not possible to separate consumption of
plastic and carton between the two companies.
As mentioned in 1.C.1, Mediq has set a new ESG strategy and are currently working on a Road Map
against 2030 to reach our ambitions.
Indicator
Waste: % of waste that is sorted for recycling.
2023
89.6%
2022
86.1%
| Mediq Norge AS | 35
Salient issue
Conflict areas / War zones
Goal :
Ensure that Mediq Norge comply to sanctions in Norwegian laws, and
stakeholder expectations in matters concerning conflict areas/War zones.
Status :
Action have been taken to ensure that Mediq Norge have no commercial
connection to Russia and Israeli companies based in illegally occupied
areas.
Goals in reporting year :
Ensure that Mediq Norge comply to sanctions in Norwegian laws, and
stakeholder expectations in matters concerning conflict areas/War zones.
Describe already implemented or planned measures to cease, prevent or mitigate negative impacts and
reasoning behind the selected measures :
As described in our EHN 2023 report, Mediq took actions when Russia invaded Ukraine to ensure that
we did not have any products in our assortment with Russian connections. A small number of items
where identified, but the relevant suppliers immediately took actions to end their collaboration with
their Russian connections. I.e by moving production to other factories.
As the sanctions against Russia has evolved, Mediq have during 2023 requested our suppliers to sign
a "Confirmation on compliance with EU sanctions and regulations regarding trade with Russia".
Concerning the ongoing Israel/Palestine conflict Mediq has assessed our suppliers. The suppliers or
manufacturing site in Israel was investigated to see if they are based within the conflict areas of Golan
heights, West Bank or Gaza. None of the suppliers are related to these areas. We will continue to
follow the situation closely.
Describe actual or expected results of measures mentioned above, as well as goals and activities for the
coming reporting year :
Mediq will continue to monitor closely on development in conflict areas / war zones.
| Mediq Norge AS | 36
OTHER ACTIONS RELATED TO MANAGEMENT OF NEGATIVE IMPACTS
Describe the enterprise's general measures to cease, prevent or mitigate negative impacts, including in the supply
chain.
3.B.1 Reduction of nature- and environmental impact
As previously described Mediq Group has relaunched our ESG strategy in 2023. A Road Map against 2030 are
under development to ensure that all Mediq countries will reach our ambitions. We have set an ambitious overall
environmental goal of working towards GHG emission neutral and circular business.
To achieve this we focus on our Products, Services, Operations and Our People.
Products:
We have intensified our collaboration with our suppliers with focus on developing our Care-to-Care selection,
with circularity as a guiding principle, rooted on the concept of the 9R’s of sustainability; rethink, refuse, reduce,
reuse, rehome, repair, restore, recycle, and rot.
This includes developing both the product and packaging.
We take our responsibility for collection and recycling of waste as member of Grønt Punkt for Packaging Material
and NORSIRK for EE-products and batteries.
Services:
We focus on supporting the sustainability transition for Healthcare providers, by offering advisory services
focusing on assortment transition towards more sustainable disposables and reusables. Services may also
include efficient assortment and inventory management that helps healthcare professionals streamline the
process of ordering medical supplies and managing hospital stock levels. Hence, avoiding rush orders or returns.
This solution not only simplifies the ordering process but also aids in organizing shelving and inventory
planning.
For complex Medical Technical Equipment we provide technical preventative maintenance, not only to ensure
safe use, but also to ensure equipment meet the expected lifespan.
Some type of equipment can be returned to Mediq, for refurbishing and to re-enter device to market. However,
this can only be done in line with governing regulations for Medical Devices to ensure the safety of the patients.
Operations:
We focus on minimizing waste generation, use of packaging material, emission in transport and energy use in
buildings.
Office buildings is classified as BREEAM NOR- Very Good. Heat pump is used for both heating/cooling. Sensor
regulated LED lighting.
Warehouse in Sweden have undergone energy mapping, and actions to reduce energy resulted in reduction of 8%
from 2022 to 2023. 100% fossil free energy are used.
Transport are done with partner with Euronorm class 6 and zero emission vehicles as far as possible. Transport
by air is strictly done when it can not be avoided, i.e for long distance transport of temperature sensitive medical
devices.
Our People:
To reach our ambitions it is essential that our people are engaged. Engaged people will be achieved by focusing
on aligning work to our strategy and by enabling, empowering and developing our employees. We focus on
training and setting personal goals aligned with our strategy.
We focus on limiting employee travels. Digital meetings have become a standard practice in society. Company
car fleet are transitioned to electric or hybrid cars. For travels within local area of headquarters of Oslo, public
transport is preferred. Office is centrally located in Oslo close to train, subway and bus. Electric bikes are made
available for our employees free of charge.
Mediq Norge are certified according to ISO14001.
| Mediq Norge AS | 37
3.B.2 Reduction of greenhouse gas emissions
Mediq Group have initiated a collaboration with ClimateParter. ClimatePartner is a sustainability-focused
company that helps businesses calculate, reduce, and offset their carbon emissions. Through their software tools
and expertise, ClimatePartner assists companies in identifying areas where they can minimize their
environmental impact and enabling organizations to take meaningful steps towards carbon neutrality.
Together with ClimateParter, Mediq are in process of setting up training and streamlining for data collection in
order to calculate Corporate Carbon Footprint for 2023. This will help Mediq get improved understanding of our
main emission drivers and further prioritize reduction measures. Some measures that we already have
implemented or are still working on are described in 3.B.1.
The calculations will include data of emissions from Scope 1, Scope 2 and for selected categories from Scope 3
according to the GHG Protocol:
- Category 1 Purchased Goods and Services: Printed products, office paper, water, external data centers,
electronic equipment, catering, the production and consumable materials, and packaging materials of 5 selected
products/product groups
- Category 3 Upstream energy
- Category 4 Upstream transport and distribution: inbound logistics
- Category 5 Waste from operations: disposal
- Category 6 Business travel: Flights, rail travel, rental and private vehicles, hotel accommodation
- Category 7 Employee travel: Employee travel, home office
- Category 9 Downstream transport and distribution: Outbound logistics
- Category 12 End-of-life treatment of sold products: Product disposal of the 5 selected products/product groups
3.B.3 Adapting own purchasing practices (sourcing)
Mediq does it upmost in regards to its purchasing practices to be a trusted long-term partner to its suppliers and
business partners. We collaborate closely with our suppliers to create predictability, transparency and efficiency,
as we believe this is the key to success for both parties.
Mediq focus on achieving qualified and reliable data of forecasts to be communicated to our suppliers, for the
suppliers to plan by. Our Demand Management team analyze demand data to optimize order predictability and
regularity. Supply Planning is responsible for executing regular orders based on well-founded demand data.
By having this close collaboration with our suppliers, we strive to have the right goods in stock at the right time
and at the same time the suppliers have predictability for optimal production- and stock planning. Hence,
avoiding to handle rush orders that put strain on suppliers, or trigger need for transport by air.
The Nordic countries in Mediq strive to have a common core of products. By acting as one party towards our
suppliers, all the Nordic countries can utilize a wide range of assortment. This allows Mediq to reduce the need to
purchase products outside of the agreed assortment, which can be challenging for the suppliers. This effort
supports Mediq to be a stable buyer, as it hopefully reduces the need for non-planned purchases which can strain
the supplier and the supplier relationship over time. Being a stable buyer is positive both for the production
planning, as well as eliminating the need for transport by air.
3.B.4 Choice of products and certifications
During 2023 Mediq have launched our own "Care-to-Care" selection. Our Care to Care selection is the result of
careful evaluation and a commitment to transparency. Our team assesses each item in this selection to ensure it
meets our sustainability standards based on the goal of becoming circular by 2050. While achieving full
circularity in healthcare may still be a distant goal, we firmly believe in taking steps now – even if they are small -
to achieve a more sustainable future. That's why our Care to Care selection criteria are rooted in the 9 R's of
sustainability: rethink, refuse, reduce, reuse, rehome, repair, restore, recycle, and rot.
Products in the Care to Care selection are labelled with a special symbol in our webshop, to help our customers
choose a more sustainable option.
You can read more about our ambitions related to products in our Care to Care selection in our ESG Strategy
(https://mediqnorge.no/om-oss/csr).
| Mediq Norge AS | 38
3.B.5 Actively support free trade union organisation and collective bargaining, or where the law does not allow it,
actively support other forms of democratically elected worker representation
Our Code of Conduct includes the following point; Freedom of Association and the Right to Collective Bargaining
(ILO Conventions Nos. 87, 98, 135 and 154).
Our suppliers are required to comply with this and also forward this requirements to its suppliers. Topic may also
be discussed with the suppliers in meetings if we suspect any risks associated with this, and in this way raise
awareness. This topic can typically be flagged as an issue in the CSR survey responses.
3.B.6 Contribution to development, capacity building and training internally and of suppliers and workers in the
supply chain
We do not contribute directly to development, capacity building and training of suppliers and workers in the
supply chain in terms of funding different programs at this time, but we work closely with suppliers which
allows us to support each other in terms of information sharing, best practices, etc.
3.B.7 Combatting corruption and bribery in own enterprise and supply chain.
Corruption is a key topic in our internal Code of Conduct. Employees are encouraged to report breaches of our
etical guidelines through standard reporting lines. In addition Mediq has a hotline to facilitate anonymous
reporting.
All employees are annually trained in our Code of Conduct through our e-training module.
3.B.8 Other relevant information concerning the enterprise’s work to reduce, prevent, and manage negative impact on
people, animals, society and the environment
We encourage you to follow Mediq Norge via LinkedIn to read news of small and large measures that Mediq does
to reduce, prevent, and manage negative impact on people, animals, society and the environment.
According to our value "Caring Hearth" we strive to help people in need. Mediq have historically been a
contributor by donating medical equipment in crisis situations across the world.
In 2023 we donated:
- Medical equipment and disposables to equip three ambulances heading for Ukraina.
- 9 pallets of Medical equipment and disposables for a hospital in Ethiopia.
- Medical products such as thermal blankets, face masks, cannulas, sanitization materials, wound care products
and more was sent to Disaster and Emergency Management Presidency in Turkey after the earthquake.
| Mediq Norge AS | 39

4
Track implementation and
results
Tracking implementation of actions and results relates to measuring the
effects of the systematic approach and own work in each step of the due
diligence process, showing whether the enterprise conducts sound due
diligence work. The enterprise needs to have procedures and routines in
place in order to uncover and critically assess own conclusions,
prioritizations and measures that have been made as part of the due diligence
process. For example, is mapping and prioritisation of salient issues done in a
scientifically sound and credible way? Does it reflect the actual conditions in
the supply chain? Do measures aimed at ceasing, preventing and reducing
the enterprise's negative impact work as intended? Is negative impact
remediated where relevant? This may apply to measures taken by the
enterprise alone or carried out in collaboration with others. The enterprise’s
experiences from working on due diligence should be used to improve
procedures and routines in the future.
4.A. Track and assess
4.A.1 Describe the a) assignment of responsibility for tracking the effect and result of measures implemented to
cease/prevent/mitigate salient risks of negative impact on people, animals, society and the environment, as well as
how the tracking is done in practice, b) who is responsible for evaluating the enterprise's implementation and work
with due diligence, and how the evaluation is done in practice.
Mediq has set up a process owner for each process. In processes where it is relevant to implement measures to
cease/prevent/mitigate salient risks of negative impact on people, society and environment, the process owner is
responsible for tracking the effect. For i.e Sourcing, who is responsible for practical performance of Supplier Due
Diligence, the reporting lines are described in 1.B.1.
Previously the Nordic Sourcing department at Mediq was responsible for tracking the effect of measures as part
of the CAPA plan set up. This is typically done by asking the supplier for documentation or to provide third party
audit reports.
With the new collaboration with Sedex, this will most likely be supported by Sedex. Which means that Sedex
might lead the tracking and follow up activities regarding the CAPA reports. As Mediq is in a transition phase
from Factlines to the new risk assessment platform Sedex the roles and Mediq responsibilities are currently
discussed and will be defined shortly.
In regards to our Mediq Own Brand products, Medeco collaborate with Amfori BSCI. As described further in
2.A.2, audits are performed when risks are suspected and follow up audits are performed after measures are taken
to verify implementation and effect.
4.A.2 Describe how the enterprise ensures that measures taken to identify, prevent and reduce negative impact
actually work
The key for Mediq to ensure that measures taken actually work is through 3rd party audits.
An example is a glove manufacturer in Malaysia that in previous audits have revealed several Non Conformities,
including use of recruitment fees for foreign workers. The dept with high interest bound the worker to the
factory, indicating forced labor. Two follow up audits was performed in 2022, documenting reimbursement of
requirement fee. A Periodic full audit from June 2023 showed that non of the previous Non Conformities was
repeated. Also, through foreign workers’ interview, it was confirmed that the facility does not retain the passport
of foreign workers. The facility has provided individual locker for workers to safe keep their passport. The locker
is located at ground floor of HR office and the facility has taken measures by installing CCTV Camera in passport
locker room for security and safety purposes. The foreign workers have option either to keep it in the designated
locker room or in dormitory.
| Mediq Norge AS | 41

5
Communicate how negative
impacts are addressed
A prerequisite for good external communication on due diligence for
responsible business conduct is that it builds on concrete activities and
results. Enterprises should make relevant documents concerning due
diligence publicly accessible, i.e. policies, codes of conduct, guidelines,
processes and activities related to identifying and handling the enterprise’s
actual and potential negative impacts on people, animals, society and
environment. Communication should include information about how the
risks have been identified and handled, as well as the effect of the
measures/activities. The Transparency Act (Åpenhetsloven) §5 requires
companies to publicly account for their human rights due diligence on an
annual basis.
5.A External communication
5.A.1 Describe how the enteprise communicates with affected stakeholders about managing negative impact
Mediq Norge has published our Policy for responsible business conduct and our annual report for Due Diligence
for Responsible Business Conduct (EHN) on our website; https://mediqnorge.no/om-oss/csr.
We have close direct dialogue with our suppliers and follow up directly to explore issue and initiate
development.
Customers have access to our published information. Customers are also informed directly. Key customers
perform annual supplier assessments which include our level of maturity related to ESG.
Mediq Group has relied on use of external competence to perform audits and to follow up on mitigating actions.
i.e. audits by SMETA.
5.A.2 Describe how the enterprise publicly communicates its own work on identifying and managing negative
impact/harm
Openness creates confidence, also regarding challenges in the supply chain. Mediq communicates it's work on
this topic in several ways, such as:
• Directly to customers in customer meetings with this topic on the agenda
• Through this report (published on our website and on social media)
5.A.3 Describe the enterprise's routines for maintaining and answering external inquiries related to the information
requirement imposed by the Transparency Act
Any inquiries from external parts about ESG and compliance to the Transparency Act is routed to the local
Norwegian ESG coordinator.
ESG coordinator involves Sourcing or other resources on Group level in case of need. As our ESG follow up of our
suppliers are risk based, we may not have all answers that are inquired.
If so, our answer will then include description of how Mediq has prioritized and why.
| Mediq Norge AS | 43

6
Provide for or cooperate to
ensure remediation when
appropriate
Once an enterprise has identified that it has caused or contributed to
negative impact on people, animals, society or the environment, the
enterprise must provide for, or cooperate in, remediation. Remediation may
involve financial compensation, a public apology or other ways to remediate
the negative impact. Another aspect of remediation is that companies should
provide for, or cooperate with legitimate complaint mechanisms, to ensure
that workers and/or local communities can raise complaints and be heard.

6.A Remediation
6.A.1 Describe the enteprise’s policy for remediation of negative impacts on people, animals, society and the
environment
Our Policy for responsible business conduct is based on template from Etisk Handel Norge.
The policy states: "If our activities are found to cause or contribute to negative impact on people, society or the
environment, we will stop the activities and seek to provide remedy. If our supplier is responsible for the
negative impact, the supplier is responsible for providing remedy."
6.A.2 If relevant, describe cases of remediation in the reporting year
In 2023 we have no examples of cases of remediation.
| Mediq Norge AS | 45

6.B. Ensure access to grievance mechanisms
6.B.1 Describe what the enterprise does to ensure that employees in own enterprise and other stakeholders,
especially impacted workers and local communities have access to whistleblowin systems and grievance
mechanisms when this is needed
Mediq have an internal speakup feedback hotline where all employees can report issues. This can be done
anonymously, if desired. Contact information is easily accessible in our Mediq Code of Conduct, to which we run
annual e-training for all employees.
Regarding grievance mechanisms in the supply chain, Mediq investigate the availability and quality of
mechanism through our SAQs.
The SAQ include questions such as:
- Wetter grievance mechanisms are a formal process, available in suitable language for workers and local
community.
- What kind of of reporting mechanisms are available (i.e through Unions, Worker Committees, Recruiter, Labor
provider or Our Supplier).
- Wetter the process protects the confidentiality of the person, protects against intimidation and retaliation.
- Wetter the remediation are documented and shared with stakeholders within reasonable timeframe.
- Training and language of people handling cases.
| Mediq Norge AS | 46

