
To Readers Of The Report
Business and the public sector have a great impact on people, society, the environment, and animals and can
both contribute positively to development, or negatively by causing harm. Businesses therefore hold a central
role in achieving UN’s Sustainable Development Goals (SDGs).
This report can be used as an account for the Transparency Act, but it has a broader scope with climate and the
environment, circular economy, and anti-corruption indicators also being included. Our members are obligated
to carry out due diligence and report annually on their work. Base level members also meet the Transparency
Act’s due diligence duty, and partially the Act’s information duty.
Responsible business conduct is the systematic effort that businesses do to identify, prevent or mitigate adverse
impacts and explain how they manage their risks of negative impact to people, society, and the environment as
well as provide remediation where this is required. Norwegian authorities expect all businesses, regardless of
their size, to carry out due diligence in accordance with the UN’s Guiding Principles for Business and Human
Rights (UNGP) and OECD’s Guidelines for Multinational Enterprises. This applies to businesses, the public
sector, and organisations.
Ethical Trade Norway’s Declaration of Principles (our Code of Conduct) covers the areas of decent work, human
rights, environment/climate, anti-corruption, and animal welfare. This report is done in full transparency and in
line with UNGP and OECD’s guidelines. The reports of all members are publicly accessible on Ethical Trade
Norway’s website.
Heidi Furustøl
Executive Director
Ethical Trade Norway
| Mediq Norge AS | 2
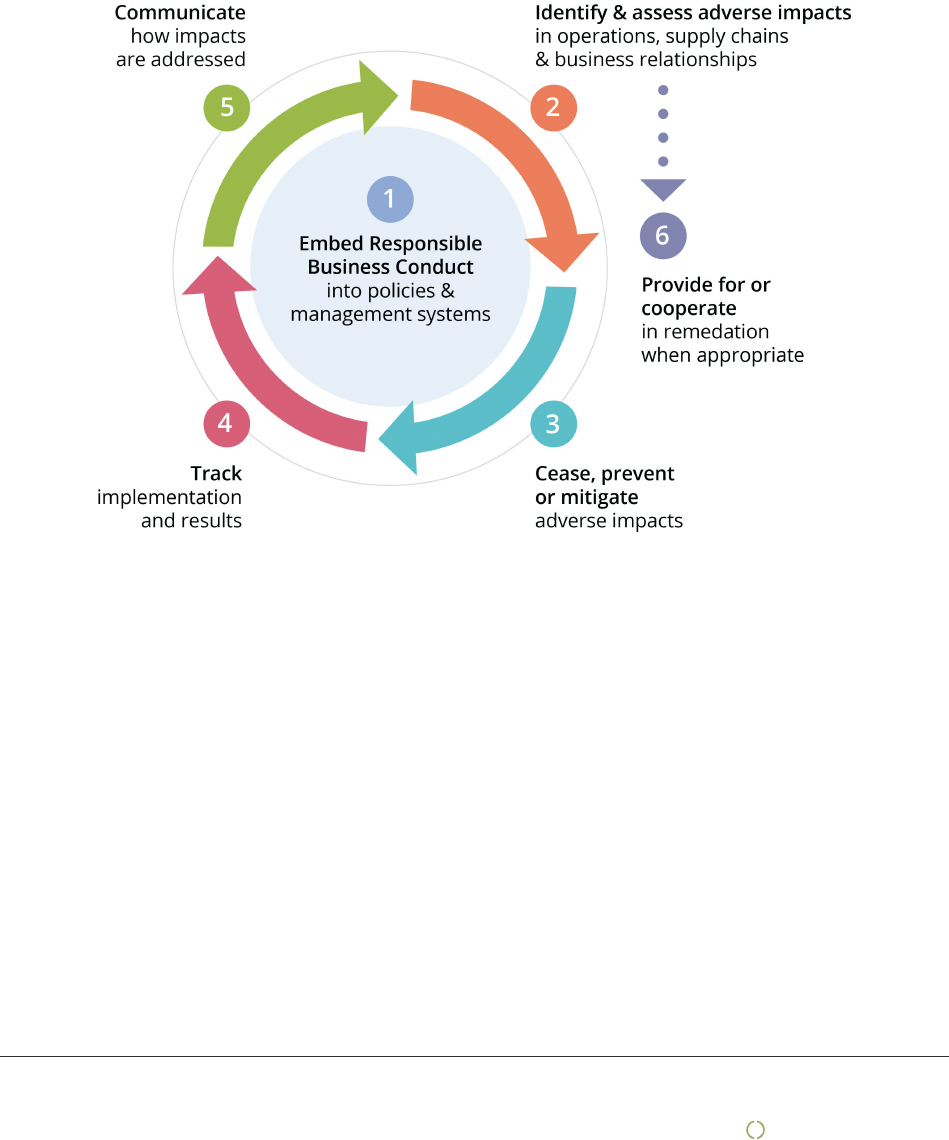
Due diligence
This report is based on the UN Guiding Principles on Business and Human Rights and the OECD model for Due
Diligence for Responsible Business Conduct.
The model has six steps that describe how companies can work for more responsible and sustainable business
practice. However, being good at due diligence does not mean no negative impact on people, planet and the
society. It means that the company is open and honest about challenges faced and shows how this is managed in
the best possible way in collaboration with its stakeholders. This report is divided in chapters following the
OECD model.
| Mediq Norge AS | 3
Preface From CEO
As a leading supplier of medical devices, Mediq Norge is naturally engaged in UN Goal 3 "Ensure healthy lives
and promote well-being for all at all ages".
We do not limit this engagement to our customers buying our products, but include everyone affected by Mediq
Norge's activity, both locally in Norway and globally in the supply chain.
We see an increased awareness of sustainability and responsible business conduct both from our customers and
suppliers, which we consider to be something very positive. The new Transparency Act came in to force in 2022
and applies to Mediq Norge.
Through our membership in Etisk Handel Norge, we have committed ourselves to continually strive to improve
conditions in our value chain.
Mediq Norge have in 2022 continued to enforce and anchor the processes and activities related to our work with
sustainability and due dilligence locally in our Norwegian organization, as well as in our Nordic Cluster and
Global Group. Mediq Norge has through our membership in Etisk Handel Norge and use of Factlines SAQ been in
a knowlegde-sharing position within Mediq Group.
While our Sourcing and Category functions are the ones closest to our suppliers, other functions within the
company such as Sales and Supply chain are also crucial for making this a collaborative effort and on top of the
agenda. We will continue our positive collaboration with our suppliers and make sure that we will do what we
can to improve both transparency and dialogue within our value chain.
Mediq Norge AS consider sustainability and responsible business conduct to be of great importance, and it is
surely aligned with our core values;
-Caring heart
-Customer drive
-Champion spirit
Trond Dahl Hansen
Administrerende Direktør, Mediq Norge AS
| Mediq Norge AS | 4

Board Signature
| Mediq Norge AS | 5

Oslo
15.02.2023
Company information and business context
| Mediq Norge AS | 6
Company information and business context
Key company information
Company name
Mediq Norge AS
Head office address
Brynsveien 14, 0667 Oslo
Main brands, products and services offered by the company
Mediq Norge sell and service Medical Devices and consumables to both public and private institutions and
companies. Suppliers are ranging from global companies with strong brands to local Norwegian companies. We
represent a number of A-brand suppliers such as: Getinge AB, Werfen, KCI Europe Holding BV, Care of Sweden
AB, Sterisol AS, Teleflex Medical Europe Ltd, Semperit Investments Asia, Ecolab AS and Boston Scientific Nordic
AB. In addition Mediq Norge represent Mediq Own Brand products, like Klinion, Curion, Absorin and Cenaman.
The Mediq Own Brands are manufactured by our sister company, Medeco.
Description of company structure
Mediq Norge AS is part of the Mediq Group with activities in 13 countries with 3000 employees.
The Mediq Group is owned by the private equity company Advent.
Within Mediq Group, Medeco is the only company that has the Manufacturer role. Medeco is the legal
Manufacturer of the Mediq Own Brands. Medeco chooses the products, contracts third-party producers and
follows up the supply chain for the Mediq Own Brand products.
Mediq Norge acts as an Importer and Distributer of a large range of products from nearly 300 different suppliers.
Medeco being one of them.
Mediq is operated in 3 European clusters, where Mediq Norge is part of the Nordic, Baltics & UK Cluster headed
by EVP Christian Kanstrup.
Trond Dahl Hansen is the Managing Director for Mediq Norge AS.
Mediq Norge is based in Oslo. Warehouse is operated by Mediq Sverige based in Kungsbacka, Sweden.
Several functions are organized pan-nordic. Such as; Supply Chain, Sourcing, Category Management, IT, HR,
Finance, Masterdata and Tender & Contract.
Turnover in reporting year (NOK)
449 290 000
Number of employees
72
| Mediq Norge AS | 7
Is the company covered by the Transparency Act?
Yes
Major changes to the company since last reporting period
Mediq Norge has in 2022 continued to strive to improve the overall business performance based on following
initiatives:
- Established a pan-Nordic organization to support the company.
- Established a pan-Nordic enterprise resource planning (ERP) system.
- Legally merged UpViser AS and Puls AS into Mediq Norge.
- Acquired Skintech AS, a acknowledged company within medical devices for estetical use.
Contact person for the report (name and title)
Kari Solhus, Quality Manager / CSR coordinator
Email for contact person for the report
kari.solhus@mediq.com
| Mediq Norge AS | 8
Supply chain information
General description of the company's sourcing model and supply chain
Mediq Norge AS is 100% owned by Mediq BV, a European market leader which proudly servers more than one
million customers.
Mediq Norge AS is a part of the Nordic cluster.
The sourcing department, which is organized as a pan-Nordic function as mentioned above, has a clear
description of all the activities and decision-making authorities.
In the Nordic Cluster we share many of the same suppliers.
The sourcing department works as a link between the supplier and the organization, and is responsible for
following up the suppliers on different levels.
Mediq Norge has a well established internal Code of Conduct. "Policy for responsible business conduct" can be
found on our website.
Based on our internal policy, we have developed a Supplier Code of Conduct which all suppliers have to commit
to. Ensuring that the Supplier signs and commits to our Supplier Code of Conduct is one of the responsibilities
that Sourcing has.
Number of suppliers with which the company had commercial relations in the reporting year
283
Comments
Commercial suppliers for Mediq Norge during the reporting year consists of 283. 242 of these suppliers are
considered tail end suppliers.
Type of purchasing/ suppliers relationships
Mediq Norge is not a Manufacturer and do not own any manufacturing sites.
80% of our supplies are purchased directly from the legal Manufacturer of the Medical Device. However, the
legal Manufacturer may do their manufacturing at both company owned factories or at contracted factories.
Often the legal Manufacturer provide articles manufactured from multiple factories and countries.
20% of our supplies are purchased from an Importer/Distributor.
List of first tier suppliers* (producers) by country
Own or joint venture
production
0%
Direct
contracting/purchas
es
80%
Purchases through
agents/intermediary/
importers/brands
20%
Other
0%
| Mediq Norge AS | 9
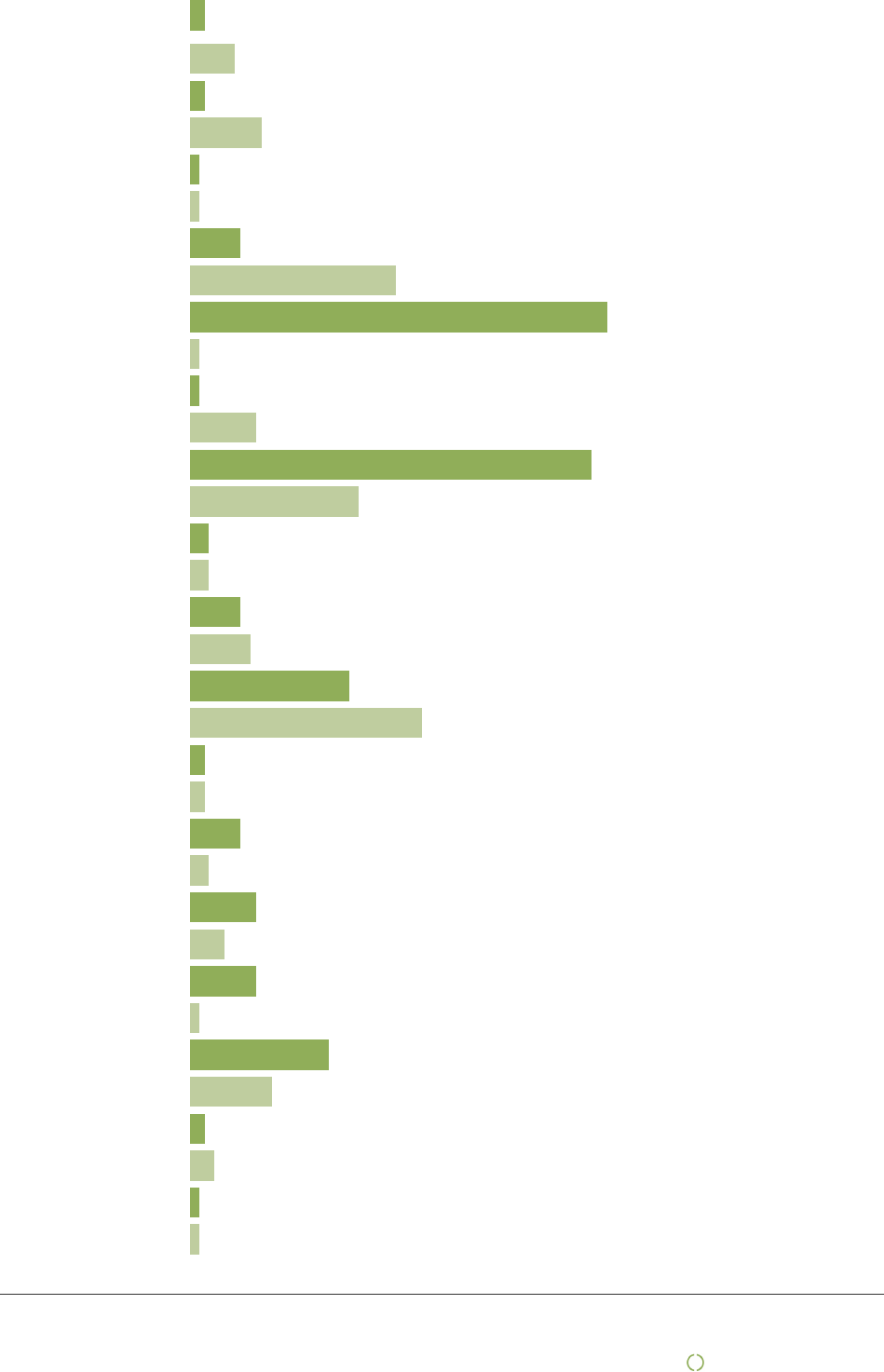
Argentina :
2
Austria :
8
Australia :
2
Belgium :
13
Brazil :
1
Belarus :
1
Canada :
9
Switzerland :
39
China :
80
Colombia :
1
Costa Rica :
1
Czech Republic :
12
Germany :
77
Denmark :
32
Dominican Republic :
3
Estonia :
3
Spain :
9
Finland :
11
France :
30
United Kingdom :
44
Georgia :
2
Hong Kong :
2
Hungary :
9
Indonesia :
3
Ireland :
12
Israel :
6
India :
12
Iceland :
1
Italy :
26
Japan :
15
Cambodia :
2
South Korea :
4
Lithuania :
1
Latvia :
1
| Mediq Norge AS | 10

Mexico :
19
Malaysia :
16
Netherlands :
21
Norway :
81
New Zealand :
4
Philippines :
1
Pakistan :
8
Poland :
18
Puerto Rico :
1
Portugal :
6
Romania :
2
Sweden :
134
Singapore :
4
Slovenia :
1
Slovakia :
11
Thailand :
5
Tunisia :
3
Turkey :
3
Taiwan :
15
USA :
78
Uruguay :
1
Vietnam :
4
South Africa :
1
Information of Country of Origin of product is collected from the supplier as we create the different stock keeping
units (SKU) in our ERP system.
Currently Mediq Norway has about 20.000 SKU.
The legal Manufacturer may do their manufacturing at both company owned factories or at contracted factories.
Often the legal Manufacturer provide articles manufactured from multiple factories and countries. Hence, the
list above of producers is larger than the number of suppliers.
State the number of workers at first tier producers that the company has an overview of, and the number of suppliers
this overview is based on:
Number of workers
5 441
| Mediq Norge AS | 11
Number of suppliers this overview is based on
21
Numbers of workers per supplier (calculated average)
260
Comments to number of workers
The numbers of workers are based on 21 of our top 150 suppliers.
Key inputs/raw materials for products or services and associated geographies
Cotton
Global
India
Pakistan
Rubber
Global
Indonesia
Thailand
Vietnam
Stainless Steel
Global
United Kingdom
Indonesia
Sweden
Plastic Global
Aluminium Global
Mediq Norge's assortment includes ~20.000 different articles. Mediq does not at this time routinely require our
suppliers to confirm the country of origin of the raw materials. This information is only collected for special
cases.
The raw materials listed here are the main raw materials for our top categories, in no particular order.
The countries and regions stated above are mainly stated due to them being large global exporters.
Is the company a supplier to the public sector?
Yes
| Mediq Norge AS | 12

Goals and progress
Process goals and progress for the reporting year
1
Goal :
Dedicate new resource in Nordic Sourcing department to have dedicated responsibility for
sustainable supply chain within end of Q2 2022.
Status :
New dedicated resource has been in place from Q2 2022.
Goal is considered completed.
2
Goal : Prepare to ensure compliance with Åpenhetsloven by 1st July 2022.
Status :
2021 report for Etisk Handel Norge was uploaded on our company webpage. Report was approved
to reach base level at EHN.
This was our main milestone to claim compliance with the Transparency Act.
3
Goal : Updated CSR data on hand by 90% of spend for the Nordic portfolio.
Status :
During the year we changed this goal to:
"Nordic Top 20 suppliers (by spend) shall be evalueted with CSR Self Assessment and action plans
created.
The status of this is that 45% of our Top 20 Suppliers has answered our survey.
Action plans for those who have answered are created.
4
Goal :
100% of our top 100 suppliers on Nordic level shall sign our updated Supplier Code of Conduct
within end on 2022.
Status : 95% of our top 100 suppliers on Nordic level has signed Supplier Code of Conduct.
5
Goal :
Annual risk analysis to identify risk countries and risk productions;
Create a Mediq High Risk List on product level.
Status : Completed.
| Mediq Norge AS | 13

Goal for coming years
1
100% of our top 100 Nordic suppliers shall sign Mediq's Code of Conduct.
2
100% of our top 20 Nordic Suppliers shall be evaluated with CSR self assessment, and a corresponding action
plan shall be created.
3
Supply Chain mapping;
1. Traceability in the supply chain on our top 10 products by spend, from our High Risk list.
2. Traceability in the supply chain on our top 10 products by spend, in total.
4
100% of our employee shall have completed our annual e-training of our Mediq Code of Conduct.
| Mediq Norge AS | 14

1
Governance and commitment to
responsible business conduct
Embedding responsible business conduct means that the company should
have strategies and plan, as well as relevant policies* and guidelines for due
diligence for responsible business conduct (hereafter due diligence) which
are adopted by management. These should comprise the enterprise’s own
operations, its supply chain and other business relationships. Effective
management systems for implementation are key to success, and due
diligence should be an integrated element in company operations. Clear
expectations from senior management are crucial, as well as clearly assigned
responsibilities within the company, for the implementation of the steps in
the due diligence process. Those involved need to know how to proceed.
Transparency about commitments the company has for itself, challenges
they are facing, and how these are managed is fundamental
1.A Policy* for own business
1.A.1 Link to publicly accessible policy for own business
https://mediqnorge.no/om-oss/csr
1.A.2 What does the company say publicly about its commitments to respect people, society, the environment and
climate?
As a leading company in our sector, there are high expectations towards Mediq. Our responsibility goes beyond
the goal of ensuring high-quality sustainable care services. Our corporate social responsibility policy is about
these main areas: the patients, the environment and the wider community.
Mediq has established a set of Mediq Code of Conduct (https://mediqnorge.no/om-oss/code-of-conduct) which
all companies in the Mediq Group need to comply according to. This document highlights Mediq`s core values in
addition to describing required business practices, quality, environment and ethical labor practices, workplace
issues as well as reporting irregularities.
Based on our Mediq Code of Conduct, Mediq has developed a Supplier Code of Conduct
(https://mediqnorge.no/om-oss/csr) that all companies in the Mediq Group use towards our suppliers. The
Supplier Code of Conduct requires that all our suppliers commits to the same principles throughout the whole
value chain. The ethical guidelines are designed to ensure that the production of our goods complies with human
rights, child labor, and labor rights.
Mediq Norway are ISO14001 certified. Our environmental management system support Mediq Norway to
minimize environmental impact by following local laws and regulations. This allows Mediq Norway to
continuously measure and improve the way our business affects the environment. Our certificates are published
on our website.
Mediq is committed to upholding ethical labor practices and procedures across all of its locations. Our
responsibility in this area includes creating awareness and understanding of human rights, employment, and
labor practices. By incorporating these principles into strategies, policies, and procedures, and living out our
values, Mediq will uphold our basic responsibilities to people, environment, and set the stage for our long-term
success. Mediq supports and respects the protection of internationally proclaimed human rights, and we strive to
ensure that we are not complicit in human rights abuses. We also uphold the freedom of association and the
effective recognition of the right to collective bargaining, the elimination of all forms of forced and compulsory
labor, and the effective abolition of child labor.
Our principles regarding the quality, environment and ethical labor practices are founded on UN and
International Labor Organization conventions as amended or restated from time to time.
Mediq Norge uses our website to communicate towards our external stakeholders how we commit to our work
doing our due diligence in our supply chain.
The website describes our CSR strategy; Strengthening the healthcare system, Patient empowerment and well-
being, Sustainable supply chain, Environmental performance and Employee engagement & well-being. In
addition our "Policy for Responsible Business Conduct" and a description of how Mediq work with Corporate
Social Responsibility towards our suppliers are published on our website.
| Mediq Norge AS | 16

1.A.3 How has the policy/commitment been developed and how is it embedded in the company?
The sender of our Mediq Code of Conduct is the CEO of the overall Mediq Group.
The policy is on the agenda from board meetings down through sales meetings, purchasing meetings, and
supplier contract.
Our Mediq Code of Conduct on corporate level are currently not describing policy for animal welfare. Hence,
Mediq Norge has a national policy document "Policy for responsible business conduct" to include this topic
(https://mediqnorge.no/om-oss/csr).
This policy document is based on resources from Etisk Handel Norge, approved by the board of Mediq Norge and
signed by Managing Director of Mediq Norge.
The Mediq Code of Conduct is part of our mandatory annual e-training module for all employees in Mediq
Group.
The onboarding process of new employees at Mediq Norge also include face to face training in CSR with the local
CSR coordinator.
Also, the company's intranet Workplace is used to communicate with all employees about the work on ethical
trade and risk in the value chain. Communication regarding our member reporting to the Ethical Trade Initiative
in Norway, as well as the risks and issues we see in markets we operate in get also shared.
Our Mediq Code of Conduct applies to all employees, officers, and directors of Mediq and governs all our
decisions and actions, whether in our offices, warehouses, in the boardroom, at customer or supplier premises or
when providing care to our patients. This Code is at the center of everything we do. It reinforces our Core Values.
We also require that all our suppliers commit to following so that the same principles are followed throughout
the value chain.
Lastly, Mediq Norway has established internal procedures in our management system for follow-up on activities
related to CSR which we take great pride in.
Mediq Norge is certified according to ISO9001 and ISO14001.
| Mediq Norge AS | 17
1.B Organisation and internal communication
1.B.1 How is the due diligence work organised within the company, and why?
Mediq's ethical guidelines are defined by Mediq Group, which all companies in the Mediq Group must fully and
wholeheartedly comply with. The management team in Norway are responsible for that the work with
responsible business conduct is carried out according to our values, with the the Managing Director being the
overall responsible for Mediq Norge.
The Nordic Mediq cluster has several joint functions. Many of the suppliers are the same across the Nordic
cluster.
The Nordic Sourcing department is responsible for the day-to-day follow up of CSR topics for the suppliers and
ethical trade within the organization.
I.e. performing the due dilligence assement. In this way we can draw synergies across the Nordic cluster.
The corporate CSR coordinator in Mediq Group has the responsibility to assist in anchoring policies and
developing processes related to CSR on Group level, and to support all business units (Mediq Countries) in CSR
matters.
The CSR coordinator in Mediq Norge has the responsibility to assist in anchoring policies at local level, ensuring
local routines related to CSR, coordinating required reporting, coordinate internal communication, as well as
promoting local requirements up to Group level.
1.B.2 How is the significance of the company's due diligence work defined and clarified for the employees through
their job description, work tasks and incentive structures?
The onboarding process at Mediq Norge include a section of CSR training conducted by the CSR coordinator in
Mediq Norge. This training is face-to-face training.
In additon, we have a mandatory e-training module to go through our Code of Conduct from Mediq Group and
why this is so important to Mediq.
The e-training module is set up for annual retraining.
CSR tasks are part of the Job Descriptions of Sourcing Specialists and CSR coordinator.
1.B.3 How does the company make sure employees have adequate competence to work on due diligence for
responsible business conduct?
Mediq support this in multiple ways, by offering our employees courses and programs which directly or
indirectly improves the way the employees conduct business such as:
• Etisk Handel Norge courses and webinars
• Direct training from Factlines
• Sharing of best practices in Supplier & Customer meetings
• Sharing of best practice internally
• Negotiation courses
• Leadership programs
• Higher educations
| Mediq Norge AS | 18

1.C. Plans and resources
1.C.1 How are the company's commitments to respect people, society and the environment embedded in strategies
and action plans?
Mediq Norge AS strives towards responsible business conduct that respects people, society and the environment.
Mediq considers responsible business conduct to be a prerequisite for sustainable development, meaning that
today’s generation get their needs covered without compromising the ability of future generations to meet their
own needs. This is in line with our Core Values: Caring Hearth, Customer Drive and Champion Spirit.
The Mediq's CSR strategy is composed of five pillars – two strategic pillars, supported by five main
transformations, and three operational pillars.
The two strategic pillars are: “Health system strengthening” and “Patient empowerment and well-being”.
The two strategic pillars are supported by 5 transformations and 3 operational pillars.
The supportive transformation pillars are: "1. We enable caregivers and patients to bring treatments home", "2.
We build ecosystems to connect patients, caregivers and health systems", "3. We educate patients and caregivers
on chronic diseases and treatments", "4. We improve access to essential medical supplies, at the right time,
quality and cost" and "5. We are committed to improving the full patient journey from prevention to care".
In addition, Mediq has three operational pillars in which we are aware of the inevitable environmental impact of
our business and strive for optimal processes to minimize our impact. "1. Sustainable supply chain", "2.
Environmental performance" and "3. Employee engagement and well-being".
The strategic pillars are aligned with the UN Sustainable Development Goals – SDG 3: ‘Ensure healthy lives and
promote well-being for people all at all ages’.
This is where Mediq can make the biggest positive impact to build a sustainable future.
(The strategy is described in our "Policy for responsible business conduct" that can be found on
https://mediqnorge.no/om-oss/csr)
Our commitment to respect people, society and the environment is directly linked you our Mediq Code of
Conduct, which is the root of our overall strategy as an organization.
Our Mediq Code of Conduct is always evolving and improving, based on the input from our market, suppliers,
customer and other organizations such as Etisk Handel Norge.
Furthermore, Mediq Norge AS are ISO 9001 and ISO14001 certified. Our Management System facilitates our
ability to consistently provide our customers with products and services that meet regulatory requirements. It
supports Mediq Norge to minimize environmental impact by following local laws and regulations. A vital part of
our Management System is to continuously measure different KPIs and improve our processes, including the
way our business affects the environment.
Mediq creates and shares CSR reports with relevant stakeholders annually. (https://mediqnorge.no/om-oss/csr)
In our Midterm CSR report for 2022, our KPIs and targets on corporate level where published.
The KPIs include:
Residual waste, scrap waste, carton consumption, plastic consumption, CO2 emission related to the transport of
parcels and pallets, electricity consumption, gas consumption, employee engagement, employee well-being, and
inclusion.
These KPIs are reported to group level on a quarterly basis.
Different improvements projects are defined to reach our goals. Examples:
-Optimize box calculations (break point between parcel and pallet)
-New plastic wrapping machines, to give more efficient usage of material
-Consolidate full case/piece picking, to give fewer outgoing parcels and increase use of outer cartons
In addition, we also supplement with KPIs on Nordic and National level. These include goals related to human
rights and labor rights in the supply chain, as described in the previous point "Goals and progress" in this report.
| Mediq Norge AS | 19

1.C.2 How is the company’s strategies and action plans to work towards being responsible and sustainable followed
up by senior management and the board?
For Mediq Norge, it is the local leadership team that are responsible for following up on the work with the
different support functions in the Nordic cluster with regards to sustainability, with the managing director being
the overall responsible for setting the agenda for Mediq Norge by making sure that:
• The achievement of the company's aims for the given year
• The company's strategy and the risks inherent in its business activities
• The compliance with legislation and regulations
Furthermore, as mentioned previously in this rapport, Mediq Norge are reliant on our core values to support
activities which the management team are overall responsible for, but also make sure to align with the support
functions in Mediq Norge to make sure that we deliver on different areas such as:
• Ensuring that our code of conduct are signed and aligned with our business partners and upheld
• Make sure that we are and remain ISO 9001 and 14001 certified by continuously working with improvements.
• Other initiatives set by other stakeholders
Nordic Sourcing Head reports monthly to Managing Director and the Norwegian management team on status
and progress, including CSR.
| Mediq Norge AS | 20

1.D Partnerships and collaboration with business relationships, suppliers in
particular
1.D.1 How does the company emphasise the importance of responsible and sustainable business conduct in its
business relationships, particularly in the supply chain?
Mediq Norge strives to abide by a responsible business conduct in line with our set policies. To achieve a
responsible business conduct in our supply chain our Nordic Sourcing organization work closely together with
our suppliers.
We require that our suppliers commit and adhere to the law and also that they have the training and tools to do
so, and that they shall be able to document their efforts to secure compliance with the local laws and our
Supplier Code of Conduct at our request. This also applies to any sub-supplier.
Mediq may terminate the relationship with any supplier, third party representative or other business partners
that fails to meet the standards in this Code after a reasonable period of time for remedying a breach.
We preform risk analysis of our product portfolio, to be able to identify our risk products and the supplier chains
connected to those.
Our minimum requirements to our suppliers include that they shall sign our Mediq Supplier Code of Conduct, or
provide us with an equal statement.
This commits the Supplier to actively communicate the content of the Supplier Code of Conduct to their workers
as well as to their suppliers. The Suppliers must at minimum require that its suppliers (our second tier supplier)
acknowledge and implement a corresponding Code of Conduct requirements.
The Supplier Code of Conduct also requires the Supplier to providing Mediq with information by responding to
supplementary questionnaire, as well as allowing Mediq or 3rd party to performing audits. (As listed in pt 6 in
Supplier Code of Conduct).
Our supplier CSR self-assessment survey is then an assessment that monitors how closely our suppliers have
implemented the Mediq Supplier Code of Conduct.
This enables insight into the various steps of the journey our products and services take from raw material to
final use. Supplier performance is scored, resulting in a CSR profile. This makes it easier for us to evaluate
suppliers and clearly identify areas for follow-up.
We collaborate with an external CSR specialized organization which provide us with digital solutions and
services for sustainable supply chain.
When the response from supplier gives reason for concern, this is flagged in the report. Prioritized concerns are
followed up with the supplier by our Sourcing department.
The results from the CSR profile report is then discussed, and sourcing department collaborates with the supplier
with any Corrective Action Preventive Action that may result from this.
To communicate Mediq policies, Mediq Norge has uploaded "Policy for responsible business conduct" and
"Supplier CSR in Mediq" to our website: https://mediqnorge.no/om-oss/csr.
Indicator
% of signing of Mediq Code of Conduct for our top 100 Nordic suppliers shall sign Mediq's Code of Conduct.
2022:
95
2021:
88
| Mediq Norge AS | 21

| Mediq Norge AS | 22

1.E Experiences and changes
1.E.1 What experiences have the company encountered during the reporting period concerning responsible business
conduct, and what has changed as a result of this?
We experience that it’s hard to get a high response rate on the CSR Supplier Survey. We are trying to collect
information about the reason for this.
Our own estimate of a potential reason is audit fatigue, due to the high numbers of companies now focusing of
this and that our suppliers has an high increase in different questionnaires they need to respond to.
We are looking into the possibility to work with another solution which makes it easier for the supplier and at the
same time gives us more information regarding the supply chain.
| Mediq Norge AS | 23

2
Defining the focus for reporting
Identify and assess the
company's impact on people,
society and environment
“Identify and assess” is about identifying the company's risk for, and actual
negative impact on, people, society and the environment, including in the
supply chain and through business relationships. As a first step the company
should get an overall risk picture, before subsequently prioritising further
mapping and measures where the risk of negative impact is the greatest, i.e.
salient issues. The company's involvemebt in the negative impact is central
to determine which measures the company should implement in the next
step of the due diligence model. Involvement of stakeholders, especially
those affected, is central when assessing risks. It is also important to consult
with stakeholders when implementing measures to manage the negative
impact.
2.A Mapping and prioritising
STATEMENT ON SALIENT ISSUES
Prioritising one or more risk areas on the basis of severity does not mean that some risks are more important than
others, or that the company should not take action on other risks, but that risks with the greatest negative impact
are prioritised first. Mapping and prioritisation are a continuous process.
2.A.1 List the company's prioritised risk of negative impact on people, society and environment. Take note that the
prioritized risk that you list in the table below will be exported to step 3 of this report, where you will be asked to
answer how you work with stopping, preventing, or reducing the negative impact.
Salient issue Related topic Geography
Labor- and human rights in production of
Medical supplies in our categories Personal
protection, Surgical & OR and Wound care &
compressions.
Forced labour
Freedom of association and
collective bargaining
Child labour
Harsh and inhumane
treatment
Occupational Health and
safety
Wages
Working hours
Marginalized populations
China
Malaysia
Pakistan
Thailand
Vietnam
Negative impact on environment from our
supply chain practice
Environment
Emission
Greenhouse gas emission
Energy
Global
Norway
Sweden
Mediq Norge is a supplier of Medical Devices. Among the sources for risk assessment that are described in pt 2A2,
we have found that the Swedwatch report “Risk-assessments for products within five categories: Medical
supplies” represents our portfolio very well.
From this report we have reviewed our different product categories and chosen to prioritize suppliers of the
following Mediq Product Categories:
-Personal protection (i.e examination gloves, surgical gloves, aprons, gowns, hair covers, caps etc)
-Surgical & OR (i.e reusable instruments like knives, forceps, scissors, clamps. Single use instruments like knives,
trocars, pins, tunnelers)
-Wound care & compression (i.e dressings, bandages, films, gauzes, plasters)
In addition Mediq has also prioritized to focus on negative impact on the environment from our supply chain
practice. Where we consider transport and packaging materials to be our biggest contributors.
| Mediq Norge AS | 25

DETERMINATION OF SALIENT ISSUES
2.A.2 Describe: a) the company's routines for mapping and identifying risk and show how the negative impact was
identified and prioritised in this period: b) eventual aspects of the company that have not been covered in this report
(product groups, own products, departments etc.) and why you not chose to prioritize these in the continued work: c)
how information was gathered, what sources were used, and which stakeholders have been involved/consulted: d)
whether you have identified areas where information is lacking in order to get an overview, and how you are planning
to proceed to collect more information/handle this.
As mentioned earlier in this report, Mediq Own Brand products are manufactured by our sister company,
Medeco, in the Mediq Group. Medeco is the legal Manufacturer of these products and the production is
outsourced to strategic manufacturing partners. Medeco follows the same framework as the Mediq Group, based
on our common Code of Conduct.
In order to have close control of the contracted manufacturers Medeco are member of Amfori BSCI, a leading
global business association for open and sustainable trade.
Amfori creates a platform which actively monitors and shares supply chain information to ensure transparent
and sustainable trade. They provide a network of independent, accredited audit companies that monitor and
evaluate factories according to the eleven BSCI principles. When not available via Amfori, Medeco audits
factories with an independent third party. Medeco performs annual due diligence on the social performance and
improvement planning of the manufacturing partners. The outcome is monitored and tracked by the
management, and when required, supplemental actions are initiated. The information is shared with our Nordic
Sourcing department, and in this way part of Responsible Sourcing program for the Nordic Cluster.
In addition to the Mediq Own Brand product provided by Medeco, Mediq Norge have about 300 external
suppliers. Some of them are the legal Manufacturer, others are Importers or Distributors. The legal Manufactures
may have multiple production sites. Some of the production sites might be their own, while others are contracted
production sites. This results in a very large amount of productions sites. For this reason we need to prioritize
and focus our work.
For our external suppliers Mediq (Nordic cluster) has chosen to do a screening of the suppliers to focus based on
type of product. As mentioned in pt 2A1 Mediq has found that the Swedwatch report “Risk-assessments for
products within five categories: Medical supplies” represents our portfolio very well. Based on information in
this report, Mediq have identified our corresponding product categories.
In this way we have created our “Mediq High Risk List”. All suppliers that deliver products within these product
categories are flagged in this list. Medeco being one of them.
In addition Mediq has chosen to broaden the focus to include 20 of our top spend suppliers.
For our annual due diligence the prioritized suppliers are subject to further questionnaires by our third party
partner.
In 2022 this resulted in 172 selected suppliers. Covering 64% of our Nordic spend. These suppliers were requested
to answer our 2022 CSR Survey, covering the following topics:
-Ethical guidelines – implemented and distributed
-Guidelines includes UN Global Compact (principle 1-10)
-Guidelines includes the corresponding ILO Conventions
-Supply chain insight and audits
-Supply chain management and risk assessment
-Corporate management systems
-Labor and human rights
-Environment, health and safety
-Anti-corruption and bribery
The questionnaire is based on recommendations from our 3rd party CSR partner, with some amendments based
on unput from our customers and other business partners.
The results are assessed and recommendations on actions are made by our 3rd party CSR partner, in the format
of a Onepager for each supplier. The sources used is listed below*.
The Nordic Sourcing department prioritize and prepare a list of follow-up activities in a CAPA plan.
The findings and corresponding CAPAs are communicated to the Suppliers, for further dialogue to solve issues.
| Mediq Norge AS | 26

Information on prioritized risks, results and assessment of collected information is shared with our Category-
and Product Managers, so that they can use the findings to further work with the product- and supplier selection.
This information is also used towards our customers either when discussing in direct meetings where CSR is a
topic, or in the surveys that we receive from them. In 2022 we have only had a limited amount of requests for
data from non-customers.
The overall rating by our 3rd party CSR partner for the suppliers was good and no severe risk was identified
among the suppliers answering the survey. The work resulted in 80 follow-up questions and actions on 38
suppliers.
In 2022 we struggled with a lower response rate, than the previous years. We have enquired response on why the
survey is not answered. And as mentioned in pt 1E1, we suspect the reason to be audit fatigue.
We are now looking internally on how we can improve the way we work and the tools we use, to get better
answering rate and input from our suppliers. And also to get better access to audit reports. This will be a part of
our work during 2023.
During 2022 we have continued the mapping the supply chain of our most high risk product categories. For some
external suppliers we have asked for audit reports from factories. We will continue this work in 2023, and also
taking one step further and identifying our most high risk raw materials.
We will do this by evaluate which raw materials and input factors are most important for our business. Identify
risk raw material, using Swedwatch risk analyze. Prioritizing and focusing on the products with the highest
spend both in total and within our high risk categories.
In addition to annual surveys, we act on information on risks that are brought to our intention by other means.
This could be from customers, business partners, news articles and peer reviewed papers.
In 2022 we have taken actions in regards to the situation in Ukraine and the updated EU regulations regarding
trade with Russia and Belarus, by following up towards our suppliers making sure they are in line with the
regulations.
In 2022 we have not performed any follow up action towards supplier that deliver products we assess as low risk.
Nor any actions towards our service providers, that are based in Norway, Sweden or Northern Europe, as we
consider these countries to be low risk. The extent of activities for these suppliers are limited to getting our
Supplier Code of Conduct signed.
Sources*:
• High risk products - The Norwegian Agency for Public and Financial Management (DFØ)
https://www.anskaffelser.no/public-procurement/socially-responsible-publicprocurement/information-about-
high-risk-products
• Transparency International Corruption Index 2021
https://www.transparency.org/en/cpi/2021
• The US Department of Labour; List of Goods Produced by Child Labor or Forced Labor 2018
https://www.dol.gov/sites/dolgov/files/ILAB/ListofGoods.pdf
• International Trade Union Confederation (ITUC) - Annual Survey of Violations of Workers Rights 2021
https://files.mutualcdn.com/ituc/files/ITUC_GlobalRightsIndex_2021_EN_Final.pdf
• US State Department: Country Reports on Human Rights Practices 2019
https://www.state.gov/reports/2019-country-reports-on-human-rightspractices/
• UN Global Sustainability Goals www.unglobalcompact.org
• Maplecroft; webinars on Human Rights and statistics https://maplecroft.com/about/webinars/watch/
• Human Rights Watch https://www.hrw.org/
• Initiative for ethical trade (NO,DK,UK) www.etiskhandel.no www.dieh.dk www.ethicaltrade.org
• The CSR Risk Check (developed and owned by MVO Nederland, funded by the Dutch Ministry of Foreign
Affairs)
https://www.mvorisicochecker.nl/en/world-map
| Mediq Norge AS | 27

ADDITIONAL SEVERE IMPACTS
2.A.3 Describe any other negative impacts on people, society and the environment that were identified in the
mapping of the business, supply chain or other business relationships during the reporting period and how these
have been handled.
Regarding the situation with Russia , Ukraine and Belarus we acted immediately in March 2022.
Our biggest suppliers (top 100) was contacted regarding the situation.
We requested information about factory location, raw material suppliers and the potential impact this will have
on deliveries to Mediq.
We found out that a few of our suppliers had connections but they could at the same time show that actions had
been made to end the collaborations.
For example action to move production to other factories.
Mediq did not see any need to take further actions.
| Mediq Norge AS | 28

3
Management of salient issues
Cease, prevent or mitigate
negative impacts
“Cease, prevent and mitigate” is about managing findings from the risk
assessment in a good way. The most salient negative impact on people,
society and the environment should be prioritised first. This does not mean
that other risks are insignificant or that they are not handled. The way the
company is involved in the negative impact is key to taking the appropriate
action. Negative impact that the company causes or contributes to must
cease, be prevented and be reduced. To address negative impact directly
linked to the company, e.g. in the supply chain, the business must use its
leverage to in¬fluence the entity causing the negative impact to cease,
prevent or mitigate it. This involves developing and implementing plans and
routines to manage risk and may require changes to the company's own
policy documents and management systems. Effective management of the
negative impact on people, society and the environment is a major
contribution to the achievement of the Sustainable Development Goals
(SDGs).

3. A Cease, prevent or mitigate
3.A.1 For each salient risk, add a goal, progress status and describe the measures you have implemented to handle
the company’s prioritized negative impact on people, society and the environment
Salient issue
Labor- and human rights in production of Medical supplies in our
categories Personal protection, Surgical & OR and Wound care &
compressions.
Goal :
Reduce negative impact on labor- and human rights.
Status :
Addressing the prioritized findings from the CSR survey to the suppliers.
Goals in reporting year :
Ensure that our requirements in our Code of Conduct is understood and
appropriately implemented by our suppliers.
| Mediq Norge AS | 30

Completed measures and reasoning :
Mediq strive to impact the practice of our suppliers through open dialogue with our suppliers.
The overall rating from the CSR survey was good (score of 78%) and no severe risk was identified
among the suppliers answering the survey.
The work resulted in 80 follow-up questions and actions on 38 suppliers.
Typical action points was; New routine implementation, Clarification of answer, Verification of
answer and Signing Supplier Code of Conduct.
Examples of new routines that required implementing is:
• Implement Ethical Guidelines/Code of Conduct for their suppliers.
• Implement systems/routines for follow-up of the supply chain.
• Establish anti-bribery and anti-corruption policy.
• Implementation of whistleblowing procedures in relation to harassment, corruption and other
illegal activities.
54 of the actions in the CAPA plan were resolved by end of 2022. (68%).
As part of our collaboration with our suppliers, suppliers are encouraged to provide us with 3rd party
audit reports to document status. In these reports we get information of breaches, how they are
handled and new status.
Example:
A manufacturer of medical device gloves was audited by ELEVATE in response to reports of alleging
indicators of forced labor on several production sites. ELEVATE conducted onsite audit December
2021, and a follow-up audit on march 2022, followed by desktop review in November 2022.
Findings:
Abuse of vulnerability; Several cases of verbal abuse and bullying from local workers to foreign
workers. Dormitory shower did not offer any privacy to workers.
Deception; Cases of work contracts in English and not in native language.
Physical and/or sexual violence; Verbal sexual harassments occurred in facility during working hours.
Withholding wages; Some employees did not receive overtime payment in certain months, due to
system relaying on facial recognition during clock out.
Excessive overtime; 15 of 15 workers sampled had worked more than 60h (max 84h). Workers
exceeded 13 days consecutively without rest.
Other; Business license missing. Cases of work permits issued to different adress. Anonymous
grievance mechanism not available for all workers. No worker representatives elected to carry out
activities relating to employees’ rights and interests. Facility missing Certificate of Completion and
Compliance. Illegal discharge of water. Missing boundary noise test. Missing competent staff to
supervise the scrubber. SDS not available in local language. Proper labelling of chemical tank missing.
Actions/Remediations are described in pt 6A2.
| Mediq Norge AS | 31

Goals and activities for the coming reporting year :
As mentioned in 2A2, we have a goal in 2023 to improve the way we work and the tools we use to get
better answering rate and input from our suppliers. And also to get better access to audit reports.
More detailed knowledge will give us a better platform to drive the dialogue for improvement with our
suppliers.
| Mediq Norge AS | 32
Salient issue
Negative impact on environment from our supply chain practice
Goal :
Reduce negative impact on environment from our supply chain practice.
Status :
Group KPIs and reporting lines have been set for various KPIs related to
environment.
Goals in reporting year :
Continue reporting on our established KPIs. Evaluate trend and effect of
various project to reach set targets. I.e:
1. We aim to decrease residual waste with a year-by-year reduction of 5%
2. We aim to decrease scrap waste production with a year-by-year
reduction of 5%
3. We aim to decrease carton consumption with a year-by-year reduction
of 2%
4. We aim to decrease plastic consumption with a year-by-year reduction
of 2%
5. We aim to decrease CO2 emission per parcel with a year-by-year
reduction of 5%
6. We aim to decrease CO2 emission per pallet with a year-by-year
reduction of 5%
Completed measures and reasoning :
All packages sent from our warehouse are packed by an automatic packing machine. The machine
measures the volume in cardboard box and cuts the sides to snugly fit to content. This reduces the
transport of dead volume and CO2 emission.
Goals and activities for the coming reporting year :
-Optimize box calculations to avoid transport of dead volumes
-New plastic wrapping machines to increase efficient use of material
-Consolidate full case/piece picking to reduce number of outgoing parcels.
| Mediq Norge AS | 33
OTHER ACTIONS RELATED TO MANAGEMENT OF NEGATIVE IMPACTS
Describe the company's general measures to cease, prevent or mitigate negative impacts, including in the supply
chain.
3.B.1 Reduction of nature- and environmental impact
Mediq Norge is certified according to ISO14001.
We take our responsibility for collection and circulation of waste. We are member of Grønt Punkt and NORSIRK.
Mediq Norge's assortment includes Medical Devices ranging from simple band aids to complex Medical
Technical Equipment like operating beds and anesthesia equipment.
We focus on sourcing biodegradable products as an alternative to plastics where this is possible. I.e like band
aids.
For complex Medical Technical Equipment we provide technical preventative maintenance, not only to ensure
safe use, but also to ensure equipment meet the expected lifespan.
Some type of equipment can be return to Mediq, for refurbishing and to re-enter device to market. However, this
can only be done in line with governing regulations for Medical Devices to ensure the safety of the patients.
Although our environmental risk assessment concludes that our negative impact is mainly from production and
transport of the products that we purchase from our suppliers, Mediq also focus on internal measures.
When Mediq Norge moved to new headquarters, the environmental aspect was listed as a requirement. Our
offices are in building classified as BREEM NOR - Very Good. The building use heat pump for both heating and
cooling. All lights are sensor regulated LED lighting.
Building is provided with free-of-charge use of electrical bicycles, that can easily be used for employees for near
by travels.
Mediq practice strict internal rules related to climate friendly transport by avoiding transport by air and
choosing transport partners with zero emission vehicels as far as possible.
Mediq have several Key Performance Indicators related to environment. This include both Group initiatives and
National initiatives.
Examples of KPIs from Supply Chain: Residual waste, Scrap waste, Carton consumption, Plastic consumption,
CO2 emission from transport of products.
Example of project we currently are working on in our Supply Chain:
-Optimize box calculations to avoid transport of dead volumes
-New plastic wrapping machines to increase efficient use of material
-Consolidate full case/piece picking to reduce number of outgoing parcels.
3.B.2 Reduction of greenhouse gas emissions
CO2 emission is a KPI at Mediq. For the transport from our warehouse to the customer, the CO2 emission pr
package and pr pallet are calculated and reported by our transport provider.
As mentioned above, Mediq have strict policies for climate friendly transport, thus avoiding transport by air. We
choose transport partners with zero emission vehicles as far as possible. We have ongoing projects to reduce
transport of dead volumes.
| Mediq Norge AS | 34
3.B.3 Adapting own purchasing practices (sourcing)
Mediq does it upmost in regards to its purchasing practices to be a trusted long-term partner to its suppliers and
business partners.
One critical procedure Mediq Norge has in place is a monthly Sales- and Operations Planning meetings (S&OP)
with key stakeholders in management.
The main purpose of these meetings is so that we can ensure that we have the right goods in stock at the right
time, which cannot be done without working closely with our suppliers. The alignment internally within Mediq
helps us to support our suppliers with qualified information regarding what they can expect in terms of
purchasing volumes and delivery dates. Hence avoiding rush orders and need for urgent transport by air.
It is an ongoing project within Mediq Norge to reduce the total number of suppliers and consolidate purchasing
volumes. Having a long tail of suppliers and products makes the tracking and maintenance of the supplier base
more complex both in terms of category management, but also with regards to ethical trade and control of the
supply chain.
To add to the point above, the work which our category managers together with our product managers put in, is
critical moving forward in regards to the assortment management and has a high priority within the Nordic
cluster. This allows Mediq to improve and define the product range of the goods needed from a supplier, thus
reducing the need to purchase goods outside of the agreed assortment, which can be challenging for the
suppliers. This effort supports Mediq to be a stable buyer, as it hopefully reduces the need for non-planned
purchases which can strain the supplier and the supplier relationship over time. Being a stable buyer is positive
both for the production planning, as well as eliminating the need for transport by air.
3.B.4 Choice of products and certifications
We do our best to convert to more eco-friendly versions of products. Ecolabelling is part of the information that
we collect when investigating for taking a new product into assortment. However, the availability of ecolabelled
Medical Devices are considered low. EU ecolabel have no criterias developed for Medical Devices. Nordic Swan
has developed criterias for only a limited segment of Medical Devices.
We stipulate in our supplier contracts that the supplier should have an active environmental policy or equivalent
and that the supplier has a responsible approach and procedures, and preferably be certified according to
ISO14001.
Reporting on certifications was included in the SAQ from 2019.
3.B.5 Actively support free trade union organisation and collective bargaining, or where the law does not allow it,
actively support other forms of democratically elected worker representation
Our Code of Conduct includes the following point; Freedom of Association and the Right to Collective Bargaining
(ILO Conventions Nos. 87, 98, 135 and 154).
Our suppliers are required to comply with this and also forward this requirements to its suppliers. Topic may also
be discussed with the suppliers in meetings if we suspect any risks associated with this, and in this way raise
awareness. This topic can typically be flagged as an issue in the CSR survey responses.
3.B.6 Contribution to development, capacity building and training internally and of suppliers and workers in the
supply chain
We do not contribute directly to development, capacity building and training of suppliers and workers in the
supply chain in terms of funding different programs at this time, but we work closely with suppliers which
allows us to support each other in terms of information sharing, best practices, etc.
| Mediq Norge AS | 35

3.B.7 Combatting corruption and bribery in own company and supply chain.
Corruption is a key topic in our internal Code of Conduct. Employees are encouraged to report breaches of our
etical guidelines through standard reporting lines. In addition Mediq has a hotline to facilitate anonymous
reporting.
All employees are annually trained in our Code of Conduct through our e-training module.
3.B.8 Other relevant information concerning the company’s work to reduce, prevent, and manage negative impact on
people, society and environment
Scorecard of risk assessment from the 3rd pary CSR partner's system is used as a tool to discuss CSR topics with
our suppliers on regular supplier meetings.
Regarding living wages; This topic is part of our Supplier Code of Conduct.
However, in 2022 our CSR survey only included questions about national minimum wages. This will be reviewed
before next survey.
| Mediq Norge AS | 36

4
Track implementation and
results
Tracking implementation of actions and results relates to measuring the
effects of the systematic approach and own work in each step of the due
diligence process, showing whether the company conducts sound due
diligence work. The company needs to have procedures and routines in place
in order to uncover and critically assess own conclusions, prioritizations and
measures that have been made as part of the due diligence process. For
example, is mapping and prioritisation of salient issues done in a
scientifically sound and credible way? Does it reflect the actual conditions in
the supply chain? Do measures aimed at ceasing, preventing and reducing
the company’s negative impact work as intended? Is negative impact
remediated where relevant? This may apply to measures taken by the
company alone or carried out in collaboration with others. The company’s
experiences from working on due diligence should be used to improve
procedures and routines in the future.

4.A. Track and assess
4.A.1 Describe the assignment of responsibility for tracking the effect of measures implemented to
cease/prevent/mitigate salient risks of negative impact on people, society and the environment, as well as how the
tracking is done in practice
Mediq has set up a process owner for each process. In processes where it is relevant to implement measures to
cease/prevent/mitigate salient risks of negative impact on people, society and environment, the process owner is
responsible for tracking the effect. Our CSR KPIs are described in our 2022 Mid year report published on our
website (https://mediqnorge.no/om-oss/csr). (Majority of these are linked to environment).
Monitoring results of our CSR KPIs are reported quarterly to Mediq Group.
In regards to the measures related to suppliers, the Nordic Sourcing department at Mediq is responsible for
tracking the effect of measures as part of the CAPA plan set up.
This is typically done by asking the supplier for documentation or to provide third party audit reports.
In regards to our Mediq Own Brand products, Medeco collaborate with Amfori BSCI. As described further in 6A2,
audits are performed when risks are suspected and follow up audits are performed after measures are taken to
verify implementation and effect.
4.A.2 Describe how the company ensures that measures taken to identify, prevent and reduce negative impact
actually work
In regards to our CSR KPIs are reported to Mediq Group. We are currently working on a dashboard tool to visually
display trends.
In regards to measures related to suppliers, audit reports are used to verify effect as described in pt 4A1.
| Mediq Norge AS | 38

5
Communicate how negative
impacts are addressed
A prerequisite for good external communication on due diligence for
responsible business conduct is that it builds on concrete activities and
results. Companies should make relevant documents concerning due
diligence publicly accessible, i.e. policies, codes of conduct, guidelines,
processes and activities related to identifying and handling the company’s
actual and potential negative impacts on people, society and environment.
Communication should include information about how the risks have been
identified and handled, as well as the effect of the measures/activities. The
Transparency Act (Åpenhetsloven) §5 requires companies to publicly account
for their human rights due diligence on an annual basis.

5.A External communication
5.A.1 Describe how the company communicates with affected stakeholders about managing negative impact
Mediq Norge have published our Policy for responsible business conduct and our annual CSR report on our
website; https://mediqnorge.no/om-oss/csr. So this information is available for all.
We have close direct dialogue with our suppliers and follow up directly to explore issue and initiate
development.
If relevant, customers are informed directly or by information published on our website.
Mediq Group has relied on use of external competence to perform audits and to follow up on mitigating actions.
I.e audits by ELEVATE.
5.A.2 Describe how the company publicly communicates its own work on identifying and managing negative
impact/harm
Openness creates confidence, also regarding challenges in the supply chain. Mediq communicates it's work on
this topic in several ways, such as:
• Directly to customers in customer meetings with this topic on the agenda.
• Through this report
• Our website
5.A.3 Describe the company's routines for maintaining and answering external inquiries related to the information
requirement imposed by the Transparency Act
Any inquiries from external parts about CSR and compliance to the Transparency Act is routed to the local
Norwegian CSR coordinator.
CSR coordinator involves Sourcing in case of need. As our CSR follow up of our suppliers are risk based, we may
not have all answers that are inquired.
If so, our answer will then include description of how Mediq has prioritized and why.
| Mediq Norge AS | 40

6
Provide for or cooperate to
ensure remediation when
appropriate
Once a company has identified that it has caused or contributed to negative
impact on people, society or the environment, the company must provide for,
or cooperate in, remediation. Remediation may involve financial
compensation, a public apology or other ways to remediate the negative
impact. Another aspect of remediation is that companies should provide for,
or cooperate with legitimate complaint mechanisms, to ensure that workers
and/or local communities can raise complaints and be heard.
6.A Remediation
6.A.1 Describe the company’s policy for remediation of negative impacts on people, society and the environment
Our Policy for responsible business conduct is based on template from Etisk Handel Norge.
The policy states: "If our activities are found to cause or contribute to negative impact on people, society or
the environment, we will stop the activities and seek to provide remedy. If our supplier is
responsible for the negative impact, the supplier is responsible for providing remedy."
6.A.2 If relevant, describe cases of remediation in the reporting year
As part of our CSR follow up of suppliers, Mediq has been provided with audit report from a manufacturer of
medical device gloves.
The audit was conducted by ELEVATE in response to reports alleging indicators of forced labor on several
production sites of the Manufacturer. ELEVATE conducted onsite audit December 2021, and a follow-up audit on
march 2022, followed by desktop review in November 2022.
The scope was to look for; abuse of vulnerability, Deception, Physical and/or Sexual violence, Withholding wages
and Excessive overtime.
Remediation activities included:
-Employees had conducted refresher training on "Abuse & Harassment Policy and Grievance Systems
Awareness"
-Shower curtains in place to provide adequate privacy
-Employment contracts were made available in workers' native language
-Procedure in place to correctly identify work attendance. So correct overtime can be paid.
-Routine in place for daily break, max working day of 10h, max working week of 60h.
-Employees trained in Grievance policy
-Wastewater pump repaired to stop illegal discharge of water
-Boundary Noise Survey was conducted
-Managment review conducted with Social Responsibility on agenda
-A certified Environemenalt Professioanl to operate scrubber hired
-MSDS available in Malay
-Chemical tanks labeled accoring to regulations
| Mediq Norge AS | 42

6.B. Ensure access to grievance mechanisms
6.B.1 Describe what the company does to ensure that workers and local communities have access to effective
grievance mechanisms when this is needed
Mediq have a speakupfeedback hotline where all employees can report issues anonymously, if desired.
Mediq are also in process to establish a whistleblower hotline for external use. We are planning to create a
reporting opportunity on our website as part of our responsible sourcing information. This is expected to be in
place latest Q1 2023.
Mediq will immediately and carefully investigate all violations brought to its attention.
| Mediq Norge AS | 43


