
Report on
Responsible Business
Conduct 2019
for Mediq Norge AS

To Readers Of The Report
Business is key for the achievement of the Sustainable Development Goals (SDGs). A well-functioning and
responsible business community contributes to sustainable development through job creation and innovative
solutions to global challenges. However, business operations can also have a negative impact on people, the
planet and the society. Members of Ethical Trade Norway have committed themselves to work with due
diligence for a more sustainable business practice.
The basis of this work is Ethical Trade Norway’s Declaration of Principles, which covers the decent work agenda,
human rights, environment/climate, anti-corruption and animal welfare. Members are obliged to report
annually on challenges they face and on measures carried out to address these. The reporting template is this
year for the first time based on the OECD due diligence model. It is new for us and new for our members. It is this
report you are currently holding in your hands. The report is publicly available on our website.
The template seeks to respond to the expectations concerning due diligence for responsible business conduct as
described in the UN Guiding Principles on Business and Human Rights and OECD Guidelines for Multinational
Enterprises. Ethical Trade Norway’s report covers essential elements of the Global Report Initiative (GRI)
reporting framework and can be used as a progress report for the Global Compact.
Heidi Furustøl
Executive Director
Ethical Trade Norway
| Mediq Norge AS | 2
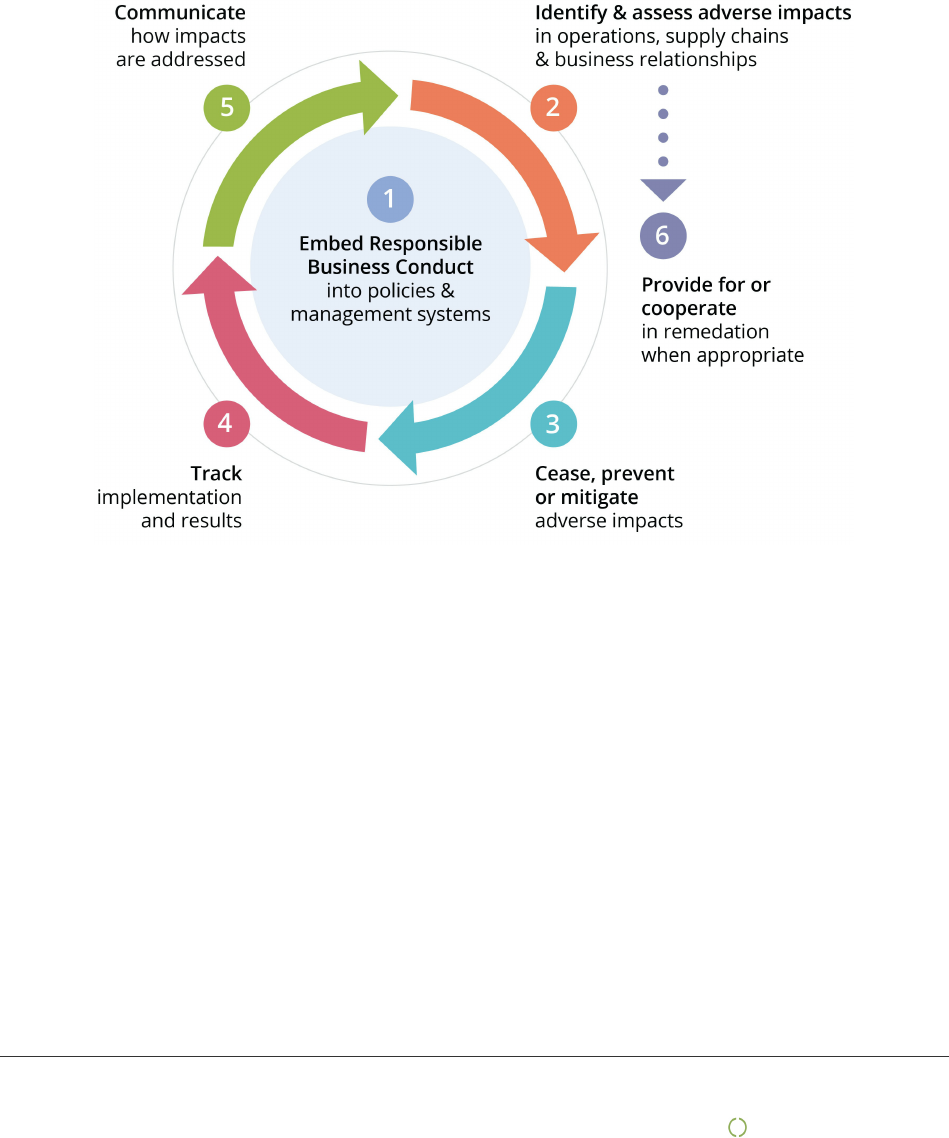
Due diligence
This report is based on the UN Guiding Principles on Business and Human Rights and the OECD model for Due
Diligence for Responsible Business Conduct.
The model has six steps that describe how companies can work for more responsible and sustainable business
practice. However, being good at due diligence does not mean no negative impact on people, planet and the
society. It means that the company is open and honest about challenges faced and shows how this is managed in
the best possible way in collaboration with its stakeholders. This report is divided in chapters following the
OECD model.
| Mediq Norge AS | 3

Preface From CEO
Foreword:
As a leading supplier of medical equipment and consumables, Mediq Norge is naturally engaged in health and
wellbeing. We do not limit this engagement to our customers buying our products, but include everyone affected
by Mediq Norge's activity, both locally in Norway and globally in the supply chain.
We see an increased awareness of issues related to ethical trade both from our customers and suppliers, which we
consider to be something very positive. We will continue our work with our internal suppliers and make sure that
we will do what we can to improve both transparency and dialogue within our value chain.
Through our membership in IEH, we have committed ourselves to continually strive to improve conditions in
our value chain. Mediq Norway has had a priority in 2019 to anchor the processes and activities related to our
work with ethical trade both on the board and our management team. While our Sourcing and Category
functions are the ones closest to our suppliers, other functions within the company such as sales and supply
chain are also crucial for making this a collaborative effort and on top of the agenda.
While we operate in Norway, we are also a part of an international company. Increased dialogue and focus on
these issues are on the agenda across our different business units. However, Mediq Norge has through the use of
Factlines SAQ with our suppliers as well as being a member of IEH for several years, a knowledge-sharing
position that we intend to use positively and constructively across our business units.
Mediq Norge AS consider ethical trade work to be of great importance, and it is surely aligned with our core
values;
Caring heart
Customer drive
Champion spirit
Trond Dahl Hansen
Administrerende Direktør, Mediq Norge AS
Company information and business context
| Mediq Norge AS | 4
Company information and business context
Key company information
Company name
Mediq Norge AS
Head office address
Brynsveien 14
Main brands, products and services offered by the company
Mediq Norway sell and service articles within 14 different categories within Medical devices and IVD offerings.
We represent the main A-brand suppliers like Coloplast, Dansac Hollister, Essity, Nutricia, Fresenius, Nestle.
Description of company structure
Mediq Norge AS is part of the Mediq Group with activities in 14 European countries. The Mediq Group is owned
by the private equity company Advent.
Trond Dahl Hansen in the Managing director for Mediq Norway. Mediq is operated in 3 European clusters, where
Mediq Norway is part of the Nordic & Baltics Cluster headed by the Nordic & Baltics EVP Christian Kanstrup.
Turnover in reporting year (NOK)
402 000 000
Number of employees
105
| Mediq Norge AS | 5
Major changes to the company since last reporting period (mergers, acquisitions etc.)
Mediq Norway has during 2019 made three major organizational changes.
• Move of warehouse from Kløfta NO to Kungsbacka SE and organization of approx. 25 people have during Q4-19
been moved from Mediq office and warehouse location at Kløfta, to Kungsbacka in Sweden.
• Mediq International BV bought company Puls AS in May 2019 AS is a leading medical equipment supplier in
Norway. The Puls organization is from 1.November 2019 integrated with the Mediq Norge organization, but is
still operating as an independent entity and continues under the Puls name.
• 16. December 2019 Mediq Norge AS and Puls AS moved together in new offices in Brynsveien 14, Oslo two
companies moved together and formed one common organization. Mediq Norge AS moved from Kløfta and Puls
AS moved from Moss, into newbuild offices in Brynsveien 14, Oslo.
Contact person for the report (name and title)
Visar Gashi, Sourcing Manager
Email for contact person for the report
visar.gashi@mediq.com
| Mediq Norge AS | 6
Supply chain information
General description of the supply chain and the company’s sourcing model
Mediq Norge AS is 100% owned by Mediq BV, a European market leader which proudly serveres more than one
million customers, Mediq Norway AS is a part of the Nordic cluster consisting of several Nordic countries.
Each country has a sourcing manager who has an overall responsibility for the sourcing activities for their
respective countries. The sourcing manager in Mediq Norway reports directly to the Nordic Category
Management & Sourcing director, and has close contact with the local Managing director.
Moreover, Mediq Norway AS purchased products from approximately 246 active trade suppliers in 2019, ranging
from global companies with strong brands to local Norwegian companies. In reality, the figure is lower due to the
recent change in warehouse location. Mediq Norway had to switch suppliers from their Norwegian entities to
their Swedish entitites due to tax purposes.
Furthermore, Mediq Norge AS is part of an international group where Own Brands is handled centrally by
Mediq's sourcing center located in the Netherlands. The Sourcing center is responsible for choosing the product,
the producers and the follow-up of the supply chain.
Mediq Norway's sourcing department, which is part of the Nordic cluster as mentioned above, has a clear RACI
chart which makes clear of all the activities or decision-making authorities across the organization. The sourcing
department works as a link between the supplier and the organization, an is responsible for following up the
suppliers on different levels. Mediq has well established Code of Conduct requirements which all incoming
suppliers have to commit to.
Number of suppliers with which the company had commercial relations in the reporting year
246
Comments to number of suppliers
Commercial suppliers for Mediq Norway during the reporting year consists 246 suppliers.
| Mediq Norge AS | 7
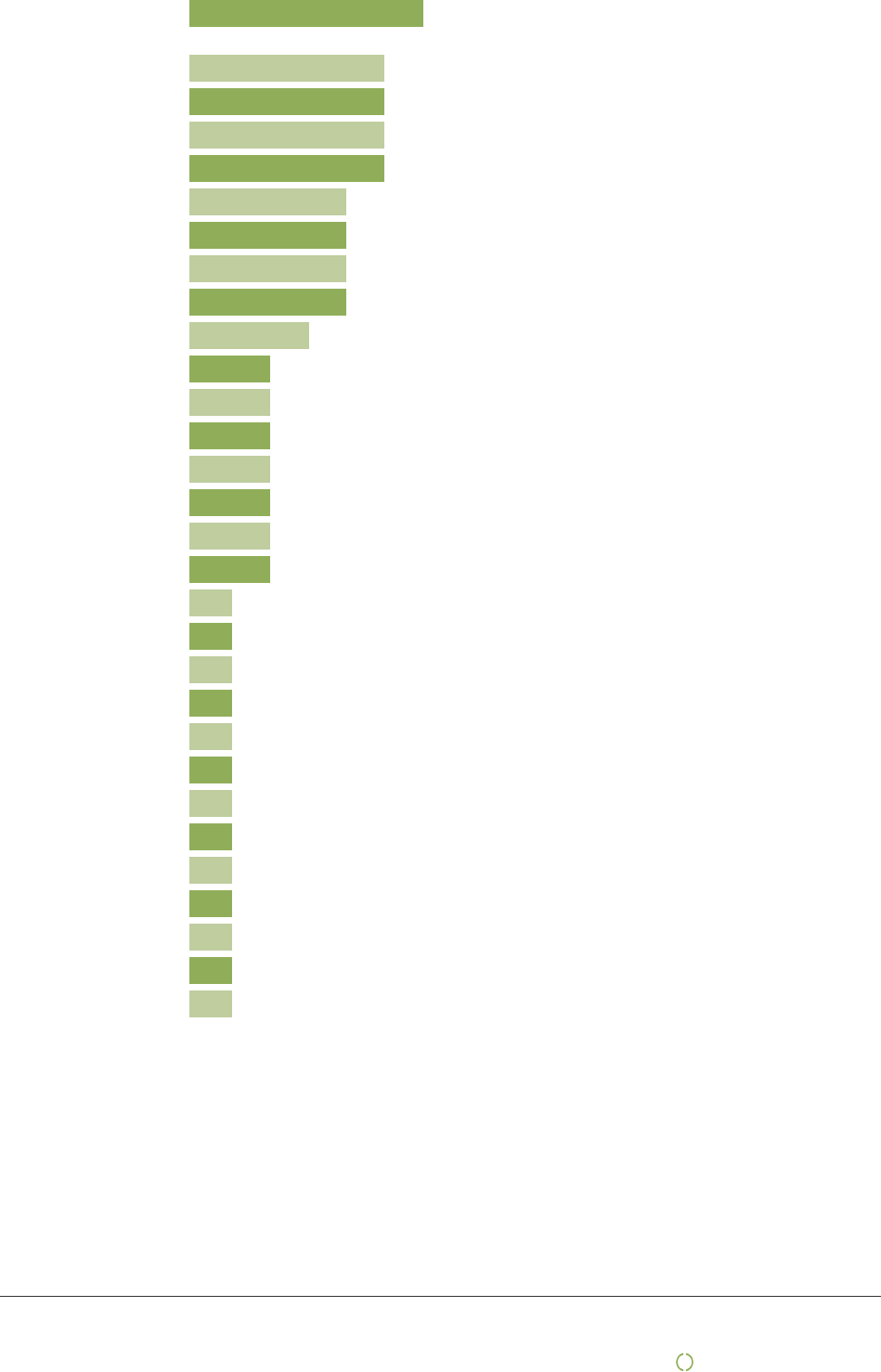
Approximate ratio by sourcing options
Mediq Norge AS is 100% owned by Mediq BV, a European market leader which proudly serves more than one
million customers, Mediq Norway Own or joint venture production:
Medeco BV, we are the legal manufacturer of the Mediq Own Brand portfolio, supporting Mediq’s patient-care
solutions across 8 categories and 14 countries. We are responsible for contracting with third-party producers to
manufacturer our portfolio of Own Brands:
Direct contracting/purchases
Mediq is a leading provider of healthcare solutions within Europe in the direct-to-patient, homecare, and
institutional segments, and that means that we need to have strong partnerships with global suppliers.
Approximately 40 suppliers stood for 80% of the Spend Mediq Norway during the reporting year of 2019.
Purchases through agents/intermediary/importers/brands
Even though direct contracting/purchases is the main source of Mediq spend, we also have some spend within
this mode of purchase above.
List of first tier suppliers (producers) by country
USA :
18
China :
16
Germany :
15
Sweden :
14
United Kingdom :
12
Netherlands :
11
France :
10
Poland :
9
Denmark :
8
Slovakia :
7
Belgium :
6
Switzerland :
6
Taiwan :
6
Italy :
6
Own or joint venture
production
15%
85%
15%
Direct
contracting/purchases
25%
75%
75%
Purchases through
agents/intermediary/importers/brands
90%
10%
Other
100%
0%
| Mediq Norge AS | 8
Mexico :
6
Spain :
5
Thailand :
5
Rzech Republic :
5
Finland :
5
Japan :
4
Norway :
4
South Korea :
4
Canada :
4
Dominican Republic :
3
India :
2
Israel :
2
Pakistan :
2
Portugal :
2
Portugal :
2
Turkey :
2
Vietnam :
2
Austria :
1
Belarus :
1
Brazil :
1
Cambodia :
1
Costa Rica :
1
Hong Kong :
1
Lithuania :
1
New Zealand :
1
Romania :
1
Singapore :
1
South Africa :
1
Tanzania :
1
Tunisia :
1
The figures above illustrate 80% of Mediq Norway's spend during the 2019 calendar year according to the
definition country of origin.
Country of origin (COO) is an international term that indicates where a product is manufactured, produced,
processed or grown. it is not to be confused with the invoice address of the country which we purchase, which is
mainly Europe
The figures below illustrate the whole spend per continent during 2019 for Mediq Norway:
Europe 62,67%
| Mediq Norge AS | 9
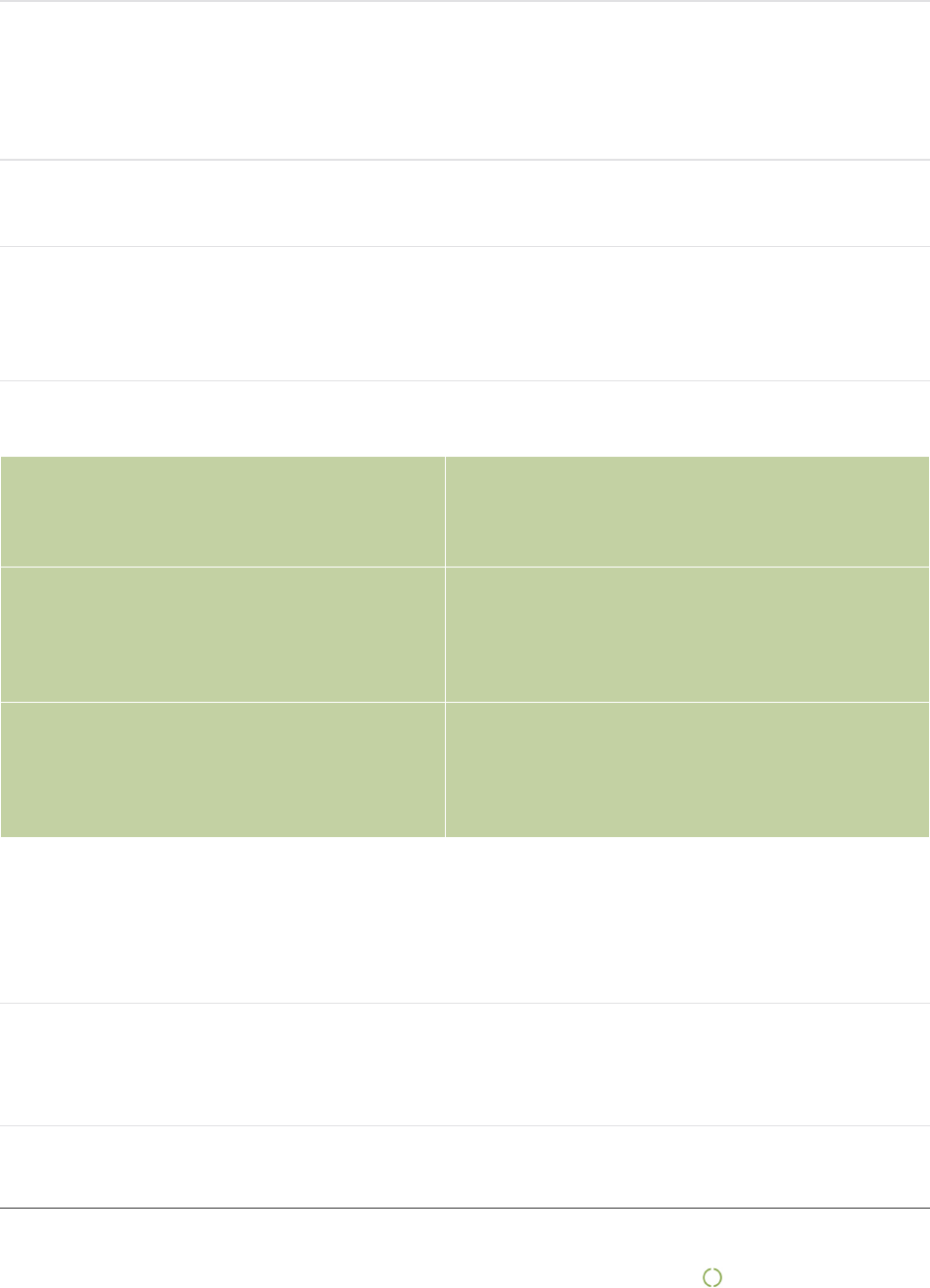
Asia 22,67%
North America 12,44%
Africa 1,33%
Oceania 0,44%
South America 0,44%
State the number of workers at first tier suppliers (producers) that the company has an overview of and the number
of suppliers this overview is based on
Number of workers
Number of suppliers
Comments to number of workers
At this point of time we do not have this kind of data from our suppliers. We are about to kick off 2020 Factlines
project and are planning to include questions related to the size of the manufacturing plant going forward.
Key inputs/raw materials and associated geographies
Cotton
Global
India
Pakistan
Rubber
Global
Indonesia
Thailand
Vietnam
Stainless Steel
Global
United Kingdom
Indonesia
Sweden
The key raw inputs above are our main raw materials for our top categories in no particular order. The countries
and regions stated above are mainly stated due to them being large global exporters. Mediq does not at this time
require our suppliers to confirm the country of origin of the raw materials unless we request it. However, we do
have great control over where our product originate from COO. This information is collected from the supplier as
we create the different SKU´s in our ERP system.
Is the company a supplier to the public sector?
Yes
| Mediq Norge AS | 10

1
Governance and commitment to
responsible business conduct
Commitment to sustainability means that the business should have relevant
policies and codes of conducts in place, as well as effective management
systems for implementing them. Central to this is the company's work with
due diligence. This means, among other things, the business need strategies
and action plans for how the company identifies and manages its risk of
negative impact on people, society and the environment, including through
business relationships and in the supply chain. Systematic management of
such risks will strengthen the company’s contribution to the Sustainable
Development Goals. Strong commitment from top-management, and clear
division of the responsibility for conducting due diligence is key. Those
involved need to know how to proceed. Sustainability should be an integral
part of business operations. Essential to this is transparency on the
company’s commitments, challenges faced and measures undertaken to
manage those challenges.
1.A Policy commitment
1.A.1 What does the company say publicly about its commitment to respect people, society and the environment?
As a leading company in our sector, much is expected of Mediq. Our responsibility goes beyond the goal of
ensuring high-quality sustainable care services. Our corporate social responsibility policy is about these main
areas: the patients, the environment and the wider community. Mediq has established a Code of Conduct that all
companies in the Mediq Group must adhere to. This requires that all our suppliers commits to the same
principles throughout the whole value chain. The ethical guidelines are designed to ensure that the production
of our goods complies with human rights, child labor, and labor rights.
Mediq Norway are ISO 14001 certified which is a set of standards established to support Mediq Norway to
minimize environmental impact by following local laws and regulations. This allows Mediq Norway to
continuously measure and improve the way our business affects the environment. Protecting the environment is
the right thing to do. We comply with all applicable environment-related rules and regulations and aspire to
adopt “best practices” in environmental procedures and standards. While our own operations have a relatively
low impact on the environment, we are alert to opportunities to reduce our environmental impact in areas where
we have the greatest influence. These have been defined as packaging, waste, and transport (input – throughput
– output). One of the pillars of Mediq’s approach to reducing environmental impact is responsible for
procurement and sourcing.
Mediq is committed to upholding ethical labor practices and procedures across all of its locations. Our
responsibility in this area includes creating awareness and understanding of human rights, employment, and
labor practices. By incorporating these principles into strategies, policies, and procedures, and living out our
values, Mediq will uphold our basic responsibilities to our people, our environment, and set the stage for our
long-term success. Mediq supports and respects the protection of internationally proclaimed human rights, and
we strive to ensure that we are not complicit in human rights abuses. We also uphold the freedom of association
and the effective recognition of the right to collective bargaining, the elimination of all forms of forced and
compulsory labor, and the effective abolition of child labor. Our principles regarding the quality, environment
and ethical labor practices are founded on the following key UN and International Labor Organization
conventions as amended or restated from time to time.
| Mediq Norge AS | 12
1.A.2 How is the commitment/policy developed and how is it anchored in the company?
The sender of our Code of Conduct is Christian Wojczewski, CEO of the overall Mediq Group. All Mediq
employees, ie management and employees in all business units have to adhere to our Code of Conduct upon
hiring, including Mediq Norway. Ethical trade is on the agenda from board meetings down through sales
meetings, purchasing meetings, and supplier contract. Mediq Norway ensures that the ethical guidelines and
commitment regarding ethical trade is communicated during the onboarding process of new colleagues.
Also, the company's intranet Workplace is used to communicate with all employees about the work on ethical
trade and risk in the value chain. Communication regarding our member reporting to the Ethical Trade Initiative
in Norway, as well as the risks and issues we see in markets we operate in get also shared.
As mentioned above, this Code applies to all employees, officers, and directors of Mediq and governs all our
decisions and actions, whether in our offices, warehouses, in the boardroom, at customer or supplier premises or
when providing care to our patients. This Code is at the center of everything we do. It reinforces our Core Values.
We also require that all our suppliers commit to following so that the same principles are followed throughout
the value chain. Any employee who fails to meet the standards in this Code, or attempts to punish a subordinate
for raising questions or for trying to follow this Code, may be subject to disciplinary actions designed to deter
wrongdoing, up to and including termination of employment. Any employee subject to this Code who is aware of
a violation and fails to report it may also face these disciplinary actions, subject to compliance with applicable
laws.
Lastly, Mediq Norway has established internal procedures in our quality system ( ISO 9001) for follow-up on
activities related to ethical trade which we take great pride in.
| Mediq Norge AS | 13

1.B Organisation and internal communication
1.B.1 How is the work with responsible business conduct organised within the company and why in this particular
way?
Mediq's ethical guidelines are defined by the Mediq HQ which all companies in the Mediq Group must fully and
wholeheartedly comply with. The management team in Norway are responsible for that the work with
responsible business is carried out according to our values, with the
the managing director being the overall responsible for Mediq Norway.
Any employee who fails to meet the standards in our code, or attempts to punish a subordinate for raising
questions or for trying to follow this Code, may be subject to disciplinary actions designed to deter wrongdoing,
up to and including termination of employment. Any employee subject to this Code who is aware of a violation
and fails to report it may also face these disciplinary actions, subject to compliance with applicable laws
1.B.2 How are employees made aware of the ways in which responsible business conduct should inform their
decisions and actions?
As mentioned in the first paragraph above, the work with responsible business conduct is mainly aligned and
described in Mediq's ethical guidelines. Each and any new employee receives this information during the
onboarding process. The values are rooted in the three following pillars: Caring Heart, Customer Drive, and
Champion Spirit.
We have transferred these pillars to business principles, work routines within quality, environment, and ethics
and behavioral patterns in the workplace. The ethical guidelines support these principles. The guidelines apply
to all our employees, directors and directors, as well as our suppliers, third-party representatives, and other
business partners.
1.B.3 How does the company make sure employees have adequate competencies to work towards implementing
responsible business conduct?
We support this in multiple ways, by offering our employees courses and programs which directly or indirectly
improves the way the employees conduct business such as:
• Negotiation courses
• Leadership programs
• Higher educations
• IEH
• Sharing of best practices in Supplier & Customer meetings
| Mediq Norge AS | 14
1.C. Plans and resources
1.C.1 How is the company’s commitment to respect people, society and the environment rooted in strategies and
action plans?
Mediq is an international company specializing in healthcare. The various markets in which we operate are not
regular markets. Governments set requirements on affordability, accessibility, and quality of care. This makes
healthcare markets highly complex and challenging.
Our commitment to respect people, society and the environment is directly linked you our code of conduct,
which is the root of our overall strategy as an organization. Our code of conduct which is included in this report
outlines this is more detail. Our code of conduct is always evolving and improving based on the input from our
market, suppliers, customer and other organizations such as Etisk Handel Norge.
Furthermore, Mediq Norge AS are also ISO 9001 which is an internationally known standard. This certification
allows Mediq to demonstrate the ability to consistently provide our customers with products and services that
meet regulatory requirements.
In addition to the ISO 9001 certification, Mediq Norway are also ISO 14001 certified which is a set of standards
established to support Mediq Norway to minimize environmental impact by following local laws and
regulations. This allows Mediq Norway to continuously measure and improves the way our business affects the
environment.
1.C.2 How is the company’s strategies and action plans to work towards being responsible and sustainable followed
up in top management and in the board?
For Mediq Norway, it is the local leadership team that are responsible for following up on the work with the
different support functions in the Nordic cluster with regards to sustainability, with the managing director being
the overall responsible for setting the agenda for Mediq Norway by making sure that:
• The achievement of the company's aims for the given year
• The company's strategy and the risks inherent in its business activities
• The compliance with legislation and regulations
Furthermore, as mentioned previously in this rapport, Mediq Norway are reliant on our core values to support
activities which the management team are overall responsible for, but also make sure to align with the support
functions in Mediq Norway to make sure that we deliver on different areas such as:
• Ensuring that our code of conduct are signed and aligned with our business partners and upheld
• Make sure that we are and remain ISO 9001 and 14001 certified by continuously working with improvements.
• Other initiatives set by other stakeholders
| Mediq Norge AS | 15

1.D Partnerships and collaboration with business associates, such as
suppliers
1.D.1 How does the company make clear in its business relationships (in particular in the supply chain) the
importance it places on responsible business conduct?
We select suppliers, third party representatives and other business partners based on their qualifications,
reliability and adherence to applicable laws and our values. We take reasonable care in selecting them
and do appropriate reviews from time to time. We require that they commit and adhere to the law and also
that they have the training and tools to do so and that they shall be able to document their efforts
to secure compliance with the local laws and our CoC at our request. This also applies to any sub-supplier.
Mediq may terminate the relationship with any supplier, third party representative or other business partners
that fails to meet the standards in this Code after a reasonable period of time for remedying a breach.
Our Code of conduct describes the key principles to ensure that we do the right thing in the right way.
Always helped, of course, by a healthy dose of common sense. Together with our vision and values, the
Code will guide our decisions and actions. This Code is applicable to all employees, officers and directors
(together, “Employees”) of Mediq and governs all our decisions and actions, whether in our offices,
warehouses, in the boardroom, at customer or supplier premises or when providing care to our patients.
This Code is at the center of everything we do. It reinforces our Core Values.
Any employee who fails to meet the standards in this Code, or attempts to punish a subordinate for raising
questions or for trying to follow this Code, may be subject to disciplinary actions designed to deter
wrongdoing, up to and including termination of employment. Any employee subject to this Code who is
aware of a violation and fails to report it may also face these disciplinary actions, subject to compliance
with applicable laws.
The Nordic identified some areas to improve and we have kicked off to great projects which will help the
Nordic cluster including Norway to improve the follow-up process of our supplier in regards to business
conduct by implementing the following:
• Aligning the cooperation with Factlines, and implementing it on a Nordic platform where we include Mediq
Norway, Sweden, Denmark, and Finland. We will together with Factlines develop and modify a new improved
standard questionnaire which also will fit the needs of each business unit.
• We will in February 2020 kick-off the exciting end-tail project together with great experts from a
consultancy company specializing in optimization of purchasing. This project will have multiple
key stakeholders from each Mediq business unit. This will improve the control and overview of
our suppliers base by reducing the number of suppliers, thus likely improving the follow-up
process moving forward.
Indicator
| Mediq Norge AS | 16
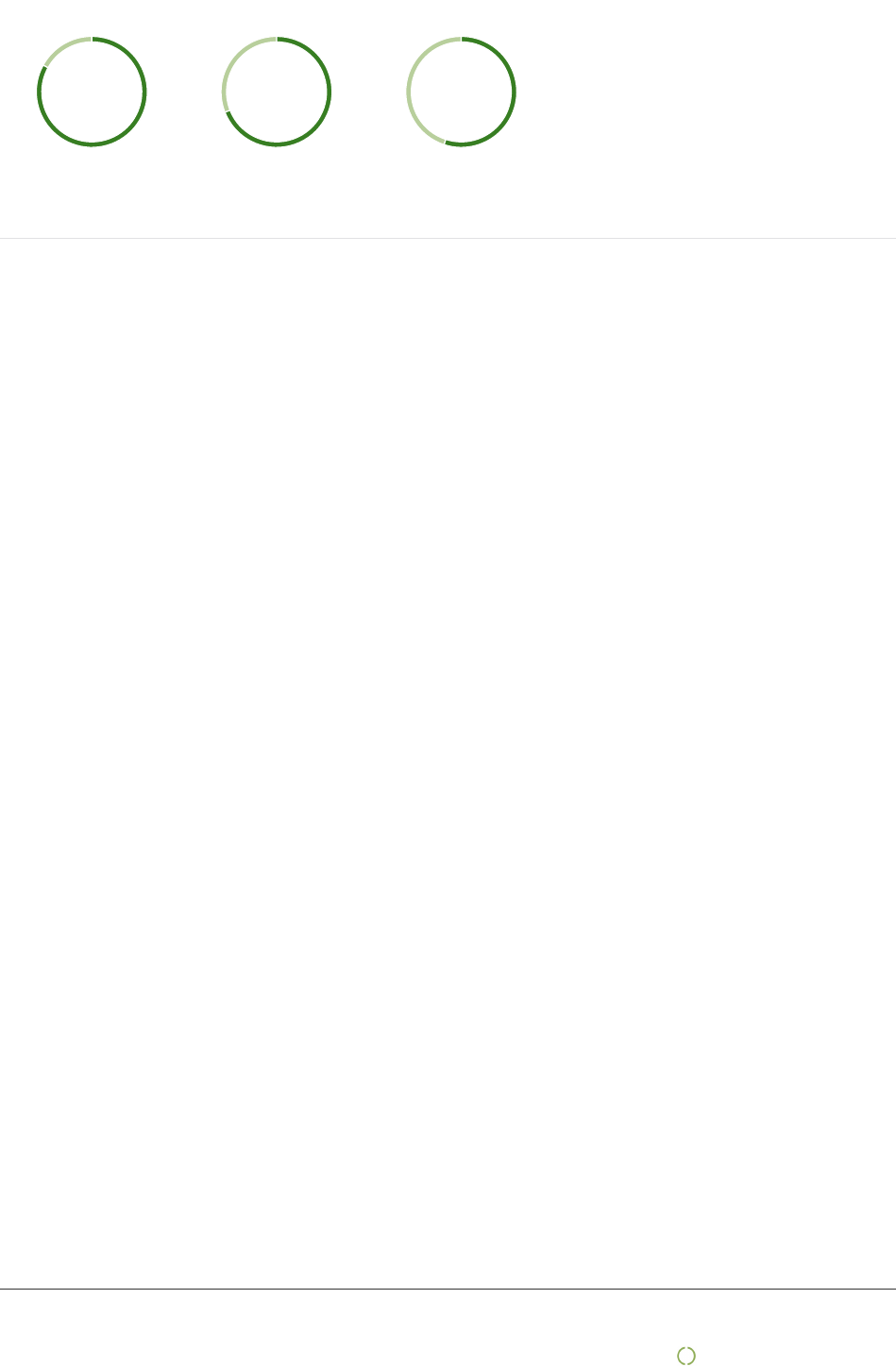
Percentage of suppliers that have accepted guidelines for suppliers
2019
17%
83%
83%
2018
31%
69%
69%
2017
45%
55%
55%
| Mediq Norge AS | 17

1.E Lessons learned and changes
1.E.1 What lessons has the company learned during the reporting period concerning sustainability, and what has
changed as a result?
The key lesson during the reporting year concerning sustainability is that we can always improve our efforts
regarding this highly important topic. Not only Mediq, but the whole supply chain going from the manufacturer
to the end customer of the products we provide.
We do our best to listen to our stakeholders, and one example of this is from our customers from the public sector
regarding the code of conduct and having this as a standard topic in meetings with our suppliers. This has been
brought up internally, and will likely be implemented as a Nordic standard in our meetings with our suppliers
where we take some time to review this topic with the suppliers on a yearly basis. The final form is to be decided.
| Mediq Norge AS | 18

2
Defining the focus for reporting
Identify and assess the
company's impact on people,
society and environment
“Identify and assess” is about identifying the company's risk for, and actual
negative impact on, people, society and the environment, including in the
supply chain and through business relations. As a first step the company
should get an overall risk picture, before subsequently prioritising measures
where the risk of negative impact is the greatest, i.e. salient issues. How the
company is involved in the negative impact is central to determine the right
actions to take. Involvement of stakeholders, especially those affected, is
central when assessing risks. It is also important to consult with stakeholders
when implementing measures to manage the negative impact.
2.A Mapping and prioritising
STATEMENT ON SALIENT ISSUES
Prioritising one or more risk areas on the basis of severity does not mean that some risks are more important than
others, or that the company should not take action on other risks, but that risks with the greatest negative impact
are prioritised first. Mapping and prioritisation are a continuous process.
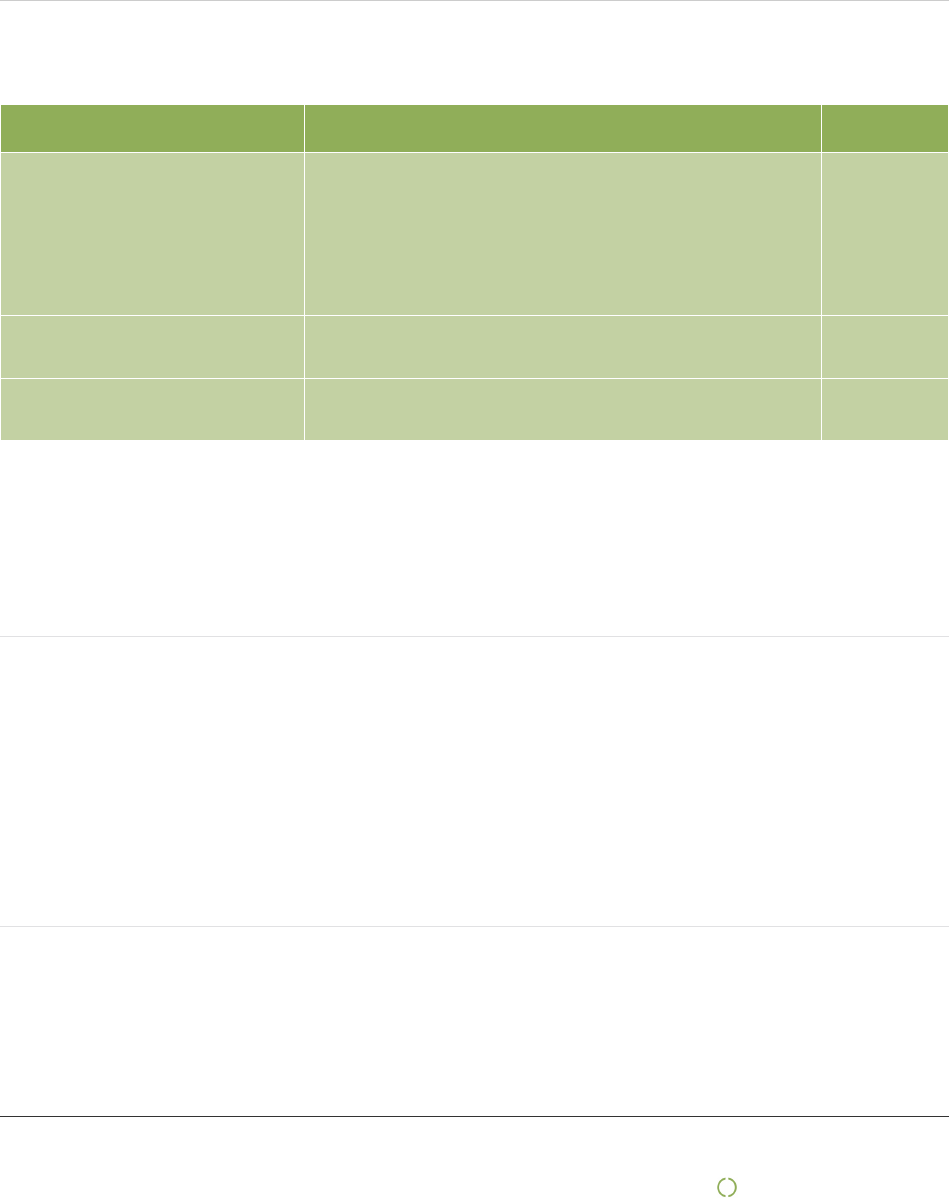
2.A.1 In the table below state the salient issues associated with the company’s activities and business relationships,
particularly in the supply chain and during the reporting period
Salient issue Related topic Geography
Examination Gloves
Forced labour
Freedom of association and collective bargaining
Occupational Health and safety
Wages
Working hours
Malaysia
Wound care & compression China
Personal protection Vietnam
We are an active members of Amfori BSCI trough Medeco BV, an external organization designed to evaluate
factories and share information.
Multiple audits have been preformed during the reporting year and Mediq Norway have been briefed regarding
the progress. We have also included our public customers and share developments.
DETERMINATION OF SALIENT ISSUES
2.A.2 Describe how the salient issues were determined, in terms of processes and sources of information, including
any input from stakeholders
Our salient issues are mainly linked to the suppliers who we suspect are in breach of our Code of conduct. There
can be multiple sources ranging from customers, business partners, news articles and also peer reviewed papers
to name a few.
| Mediq Norge AS | 20

ADDITIONAL SEVERE IMPACTS
2.A.3 Identify any severe impacts on people, society and the environment that occurred or were still being addressed
during the reporting period, but which fall outside of the salient issues, and explain how they have been addressed.
These matters are covered in our audit reports.
| Mediq Norge AS | 21

3
Management of salient issues
Cease, prevent or mitigate
negative impacts
“Cease, prevent and mitigate” is about managing findings in a way that
contributes to a sustainable and responsible business conduct. The most
severe negative impact on people, society and the environment should be
prioritised first. This does not mean that other risks are less important or that
they are not handled. The way the company is involved in the negative
impact is central to taking the right action. Negative impact that the
company causes or contributes to must cease, and the business must work to
prevent and mitigate such risk. To address negative impact directly linked to
the company, e.g. in the supply chain, the business must use its leverage to
influence the entity causing the adverse impact to cease, prevent or mitigate
it. This involves developing and implementing plans and routines to manage
risk and may require changes to the company's policy documents and
management systems. Effective management of the negative impact on
people, society and the environment is a major contribution to the
achievement of the SDGs.
3. A Cease, prevent or mitigate
3.A.1 For each salient issue in your supply chain, add a goal, status and describe specific actions and progress made
in the reporting year
Salient issue
Examination Gloves
Goal :
Status :
Objectives in reporting
year :
Actions :
| Mediq Norge AS | 23

Salient issue
Wound care & compression
Goal :
Status :
Objectives in reporting
year :
Actions :
| Mediq Norge AS | 24

Salient issue
Personal protection
Goal :
Status :
Objectives in reporting
year :
Actions :
| Mediq Norge AS | 25
Other actions related to management of negative impact:
Describe general actions to cease, prevent or mitigate negative impacts, including in your supply chain
3.B.1 Reduction of environmental and climate footprint
As a leading company in our sector, much is expected of Mediq. Our responsibility goes beyond the goal of
ensuring high-quality sustainable care services. Mediq Norge AS is a supplier who takes care of it's ethical and
environmental obligations seriously. We are therefore a member of Green Point Norway and the and are
environmentally certified according to NS-ISO 14001: 2004.
The main elements of the standard are:
• Environmental policy
• Planning
• Implementation and operation
• Control and repair
• Management Evaluation
3.B.2 Adapting own purchasing practices (sourcing)
Mediq does it upmost in regards to its purchasing practices to be a trusted long-term partner to its suppliers and
business partners.
One critical procedure Mediq Norway has in place is monthly a Sales- and Operations Planning meetings (S&OP)
with key stakeholders in management. The main purpose of these meetings is so that we can ensure that we have
the right goods in stock at the right time, which cannot be done without working closely with our suppliers. The
alignment internally within Mediq helps us to support our suppliers with quality information regarding what
they can except Mediq in terms of purchasing volumes when needed.
It is an ongoing project within Mediq Norway to reduce the total number of suppliers and consolidate purchasing
volumes. Having a long tail of suppliers and products makes the tracking and maintenance of the supplier base
more complex both in terms of category management, but also with regards to ethical trade and control of the
supply chain.
To add to the point above, the work which our category managers together with our product managers put in is
critical moving forward in regards to the assortment management and has a high priority within the Nordic
cluster. This allows Mediq to improve and define the product range of the goods needed from a supplier, thus
reducing the need to purchase goods outside of the agreed assortment, which can be challenging for the
suppliers. This effort supports Mediq to be a stable buyer, as it hopefully reduces the need for non-planned
purchases which can strain the supplier and the supplier relationship over time.
3.B.3 Choice of product design and of raw materials
We do our best to convert products lines from suppliers over to more eco-friendly versions. We stipulate in our
supplier contracts that the supplier should have an active environmental policy and is certified according to ISO
14001 or equivalent and that the supplier has a responsible approach and procedures. Reporting on certifications
was included in the SAQ from 2019.
| Mediq Norge AS | 26
3.B.4 Actively support free trade union organisation and collective bargaining, or where the law does not allow it,
actively support other forms of democratically elected worker representation.
The Mediq group, including Mediq Norway AS has a strong Code of Conduct which is written based on our core
values. in this area specifically, our principles are founded on the following key UN and International Labor
Organization convention.
• Freedom of Association and the Right to Collective Bargaining (ILO Conventions Nos. 87, 98, 135 and
154)
Furthermore, we use the third party partner Factlines as support, which allows us to focus follow-up work on the
part of the value chain where the risk of human rights violations is greatest.
With that said, we can always improve our active and more direct support regarding free trade union
organizations.
3.B.5 Contribute to development, capacity building and training of suppliers and workers in the supply chain:
We do not contribute directly to development, capacity building and training of suppliers and workers in the
supply chain in terms of funding different programs at this time, but we work closely with suppliers in which is a
function which allows us to support each other in terms of information sharing, best practices, etc.
We can definitely improve in this area and appreciate IEH shedding light on this topic in this manner, which
allows us to bring this forward.
3.B.6 Other plans and measures taken to deal with salient issue
As mentioned above, this is an area where we can improve for the next reporting year.
| Mediq Norge AS | 27

4
Track implementation and
results
Tracking implementation of actions and results is key to the company’s due
diligence process. For example, is the identifying and prioritisation of salient
issues done in a scientifically sound and credible way? Does it reflect real
conditions in the supply chain? Do measures aimed at ceasing, preventing
and reducing the company's negative impact work as intended? Is negative
impact remediated where relevant? This may apply to actions taken by the
company alone or carried out in collaboration with others. Companies must
have procedures and systems to track their implementation and results in
order to assess them. The company’s experience with due diligence is used to
improve processes and results in the future.
4.A Monitoring and assessment
4.A.1 Describe responsibilities and procedures within the company for tracking performance with respect to due
diligence activities
In regards to our relationship with third-party suppliers, we take several steps. We are an active member of
Amfori BSCI, an external organization designed to evaluate factories and share information. Potential partners
are subject to a rigorous selection and quality
criteria as part of the overall Mediq Sourcing Policy.
Furthermore, our quality team in Holland performs independent audits on our suppliers annually on a risk-
based assessment, where we get the opportunity to cooperate closely with the suppliers to uncover weaknesses
and flaws. Based on the findings we create a corrective and preventive action plan which can cover topics going
from health and safety to working hours. The Capa consist of four key elements which the supplier must submit
back to Mediq's quality team:
• Completed Date
• Audit Category
• Preventive / Corrective Action
• Actions completed
4.A.2 Describe how the company evaluates the effect of its own efforts, or those made by suppliers (and other
business relations), to identify, prevent and mitigate salient issues
As mentioned above, our quality team in Holland performs independent audits on our suppliers annually on a
risk-based assessment, where we get the opportunity to cooperate closely with the suppliers to uncover
weaknesses and flaws. Based on the findings we create a corrective and preventive action plan which can cover
topics going from health and safety to working hours. The Capa consist of four key elements which the supplier
must submit back to Mediq's quality team:
• Completed Date
• Audit Category
• Preventive / Corrective Action
• Actions completed
The suppliers receive follow up requests from Mediq regarding salient issues and are urged to solve the
complaints. Mediq have preformed multiple audits in the reporting year which leads to wider experience and
lessons learned over time.
| Mediq Norge AS | 29

5
Communicate how impacts are
addressed
Relevant external communication on company due diligence for responsible
business conduct needs to build on specific activities and results. This
include external communication of policies and codes of conduct, or
processes and activities related to identifying and managing the company's
actual and potential negative impact on people, society and the environment.
Communication should also include findings, effects and results of concrete
actions or activities.

5.A External communication
5.A.1 Describe how the company communicates with affected stakeholders when managing its salient issues
• Supplier self-assessment questionnaires based on Mediqs code of conduct
• direct supplier dialogue to confirm support and follow-up with suppliers
• Direct follow-up activities to explore issues and initiate development.
First steps taken in 2019 – plan to expand in 2020
5.A.2 Describe how the company communicates publicly about its own work on identifying and management of
salient issues
Openness creates confidence, also regarding challenges in the supply chain. Mediq communicates it's work on
this topic in several ways, such as:
• Directly to customers in customer meetings with this topic on the agenda.
• Through this report
• Our website
| Mediq Norge AS | 31

6
Provide for or cooperate to
ensure remediation when
appropriate
Once a company has identified that it has caused or contributed to negative
impact on people, society or the environment, the company must provide for,
or cooperate to ensure remediation. Remediation may involve financial
compensation, a public apology or other ways to remediate the negative
impact. When appropriate, companies should provide for or cooperate with
legitimate remediation mechanisms through which impacted stakeholders
and rights holders can raise complaints.

6.A Remediation
6.A.1 Describe the company’s policy for remediation of negative impacts on people, society and the environment
This point is to be further developed in the upcoming reporting year, even though we do preform audits which
we have explained in the earlier chapters. We require corrective actions from the suppliers if needed.
6.A.2 Describe cases of remediation in reporting year, if relevant
As mentioned above.
| Mediq Norge AS | 33

6.B Secure access to grievance mechanisms
6.B.1 Describe what the company does do to ensure that workers and communities have access to effective
remediation mechanisms, when appropriate:
Complaints may be made without the risk of steps being taken against the employee who reports the complaint.
Mediq will immediately and carefully investigate all violations brought to its attention.If this is not possible or
desirable, it can be reported (anonymously if desired) via
the integrity procedure. The free phone number and website to report irregularities are the following:
Belgium 0800-71365 www.speakupfeedback.eu/web/trbtap/be 67382
Denmark 80885638 www.speakupfeedback.eu/web/trbtap/dk 02884
Estonia 800 0044 208 www.speakupfeedback.eu/web/trbtap/ee 18559
Finland 08001-13031 www.speakupfeedback.eu/web/trbtap/fi 92280
France 0800-908810 www.speakupfeedback.eu/web/trbtap/fr 16007
Germany 0800-1801733 www.speakupfeedback.eu/web/trbtap/de 75390
Hungary 0680981359 www.speakupfeedback.eu/web/trbtap/hu 96070
Latvia 8000 2490 www.speakupfeedback.eu/web/trbtap/lv 74222
Lithuania 880090006 www.speakupfeedback.eu/web/trbtap/lt 59708
Netherlands 0800 0222931 www.speakupfeedback.eu/web/trbtap/nl 72330
Norway 800-18333 www.speakupfeedback.eu/web/trbtap/no 18669
Sweden 020-798813 www.speakupfeedback.eu/web/trbtap/se 62220
Switzerland 0800-561422 www.speakupfeedback.eu/web/trbtap/ch 51587
USA 1-866-2506706 www.speakupfeedback.eu/web/trbtap/us 44638
| Mediq Norge AS | 34

